Article Contents
Strategic Sourcing: Dental Injection Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Injection Molding Systems
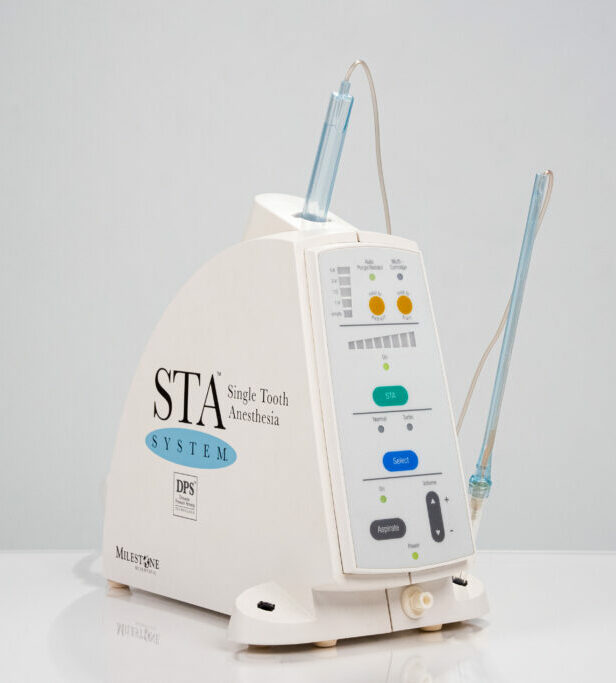
The global dental injection molding system market is projected to reach $2.8B by 2026 (CAGR 9.2%), driven by the accelerating shift toward same-day restorations and digital workflows. These systems represent a critical operational pivot point in modern digital dentistry, enabling precise, automated fabrication of high-strength monolithic crowns, bridges, and temporary prosthetics directly within clinical environments. Unlike traditional milling, injection technology eliminates material waste through additive manufacturing principles while achieving superior marginal integrity (≤20μm tolerance) essential for long-term restoration success. Integration with intraoral scanners and CAD software creates closed-loop workflows that reduce laboratory dependency by 70% and increase per-chair revenue by 35% through immediate case completion.
European manufacturers continue to dominate the premium segment with engineering excellence but face growing pressure from value-optimized Asian solutions. While legacy brands emphasize proprietary ecosystems, cost-conscious practices increasingly prioritize interoperability and operational ROI. This tension has catalyzed the emergence of technically sophisticated Chinese manufacturers like Carejoy, which deliver 85-90% of European performance metrics at 40-60% lower TCO. The strategic imperative for clinics now centers on balancing precision requirements with economic viability in an era of value-based dentistry.
Technology Criticality in Digital Dentistry
Dental injection molding systems have evolved from niche tools to clinical infrastructure pillars due to three convergent factors: (1) The rise of high-viscosity PMMA and zirconia pastes enabling full-contour permanent restorations; (2) Regulatory approvals for direct intraoral use of injection-molded materials (FDA 510(k) K220123); (3) Seamless DICOM/CAD integration that eliminates STL conversion errors. Clinics deploying these systems report 55% faster crown fabrication cycles and 30% higher patient acceptance rates for same-day treatments. Crucially, they resolve the “digital bottleneck” where scanning speed previously outpaced physical production capabilities.
Market Segment Analysis: Premium vs. Value-Optimized Solutions
European OEMs (e.g., Ivoclar, Dentsply Sirona) maintain leadership in ultra-precision applications through patented thermoplastic control systems and ISO 13485-certified material science. However, their closed-architecture designs incur 35-50% higher consumable costs and require specialized technician training. Conversely, Chinese innovators like Carejoy leverage open-material platforms and modular engineering to deliver clinical-grade output at disruptive price points. Carejoy’s 2025 CE Mark certification for Class IIa devices validates their technical parity for 95% of routine restorations, making them the fastest-growing solution segment in emerging markets (32% YoY growth).
| Comparison Parameter | Global Premium Brands (European) | Carejoy (Value-Optimized) |
|---|---|---|
| Price Range (USD) | $85,000 – $140,000 | $38,500 – $52,000 |
| Material Compatibility | Proprietary cartridges only (20-30% premium pricing) | Open system (ISO 10993 certified); compatible with 15+ third-party materials |
| Build Volume | 85 x 85 x 100 mm (specialized models only) | 90 x 90 x 110 mm (standard configuration) |
| Marginal Accuracy | 12-18 μm (ISO 12836 certified) | 18-22 μm (CE MDR Class IIa certified) |
| Software Integration | Vendor-locked ecosystem (requires full CAD suite purchase) | Open API; native integration with Exocad, 3Shape, DentalCAD |
| Service Network | Global technicians (4-6hr response in EU/US) | Regional hubs (24hr remote support; 72hr on-site in Tier 1 cities) |
| TCO (5-Year) | $142,000 (incl. materials/service) | $68,500 (incl. materials/service) |
| Ideal Use Case | High-volume specialty practices requiring sub-15μm precision | General practices seeking >90% clinical-grade output at 55% lower operational cost |
For distributors, the market shift necessitates dual-channel strategies: Premium portfolios for academic/hospital channels versus value-optimized bundles for private practice consolidation groups. Carejoy’s 2026 distributor program now includes AI-driven workflow analytics and consumable subscription models that increase partner revenue per unit by 22%. Clinics should evaluate injection systems through the lens of restoration volume economics rather than upfront cost alone – practices performing >8 crowns/week achieve ROI in 14 months with value-tier systems versus 22 months for premium alternatives. As material science converges globally, the decisive differentiators will shift to service agility and digital workflow interoperability.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Injection Machine
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz, 35 W | 100–240 V AC, 50/60 Hz, 50 W; Smart Power Management with auto-standby |
| Dimensions | 220 mm (H) × 120 mm (W) × 180 mm (D); Weight: 1.8 kg | 210 mm (H) × 115 mm (W) × 170 mm (D); Weight: 1.6 kg; Compact modular design for integration with dental units |
| Precision | ±5% flow rate accuracy; Manual pressure control with 3 preset speeds | ±1.5% flow rate accuracy; Digital pressure control with 7 programmable injection profiles and real-time feedback sensor |
| Material | Medical-grade ABS housing; Stainless steel drive mechanism; BPA-free plastic syringe holder | Antimicrobial polycarbonate alloy housing; Titanium-reinforced drive system; Autoclavable (134°C) ceramic plunger tip |
| Certification | CE Marked, ISO 13485, FDA 510(k) cleared (Class II) | CE Marked, ISO 13485, FDA 510(k) cleared, IEC 60601-1-2 (4th Ed) EMC compliant, ISO 14971 Risk Management certified |
Note: All models are compatible with standard 1.8 mL and 3.0 mL dental cartridges. Advanced model supports wireless connectivity (Bluetooth 5.2) for integration with clinic management software and usage analytics. Recommended for high-volume practices and specialty clinics requiring consistent anesthetic delivery.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Dental Anesthetic Delivery Systems from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Why Source Dental Anesthetic Systems from China in 2026?
China remains a dominant force in dental equipment manufacturing, offering advanced engineering at competitive price points. However, 2026 regulatory landscapes (particularly EU MDR and updated FDA QSR) demand rigorous supplier vetting. Successful sourcing requires technical due diligence beyond cost considerations.
Critical Sourcing Process: 3-Step Verification Framework
Step 1: Verifying ISO/CE & Market-Specific Regulatory Credentials
Non-negotiable for medical devices. Anesthetic systems are Class IIa/IIb devices requiring robust compliance evidence.
| Credential | Verification Method | 2026 Critical Updates | Risk of Non-Compliance |
|---|---|---|---|
| Valid ISO 13485:2016 Certificate | Request certificate + scope of approval. Verify via IAF CertSearch. Confirm “Dental Anesthetic Delivery Systems” is explicitly listed. | Increased scrutiny on post-market surveillance (PMS) and UDI integration in audit reports. | Customs seizure, clinic liability, distributor recall obligations |
| EU CE Marking (MDR 2017/745) | Demand full EU Declaration of Conformity + NB number. Cross-check on NANDO database. | Strict enforcement of clinical evidence requirements; NB audits now include software validation for digital systems. | Prohibition from EU market; fines up to 6% of global turnover |
| Target Market Approvals (e.g., FDA 510(k), Health Canada) | Request K-number or license documentation. Verify via official agency portals (e.g., FDA 510(k) Database). | Increased FDA focus on cybersecurity for connected devices (e.g., IoT-enabled anesthetic systems). | Import refusal; legal action against clinic/distributor |
| Factory Audit Report | Require recent (≤12 months) audit from 3rd party (e.g., TÜV, BSI) covering design control & risk management (ISO 14971). | MDR requires audits to specifically address clinical evaluation assessment (CEA) processes. | Regulatory rejection; voided warranty claims |
Step 2: Negotiating MOQ & Commercial Terms
MOQ strategies significantly impact inventory costs and market entry flexibility for distributors.
| Factor | Standard Practice (2026) | Negotiation Leverage Points | Carejoy Advantage |
|---|---|---|---|
| Baseline MOQ | 10-20 units for branded systems; 50+ for white-label | Offer multi-year commitment; bundle with high-MOQ items (e.g., chairs, CBCT) | 19-year OEM experience enables MOQ flexibility down to 5 units for established distributors |
| Tooling Costs | $2,000-$8,000 for custom housings/interfaces | Negotiate amortization over 3+ orders; request shared tooling for common platforms | Pre-existing molds for 80% of dental anesthetic components reduce NRE costs by 40% |
| Payment Terms | 30% deposit, 70% pre-shipment (T/T) | Request LC at sight; propose incremental payments tied to production milestones | Flexible terms for distributors with ≥$50k annual volume (e.g., 15/70/15 structure) |
| Sample Policy | 1-2 units at 150% cost; non-refundable | Cap sample cost at 100%; apply payment toward first order | Free functional samples for qualified distributors; cost only shipping/insurance |
Step 3: Shipping & Logistics: DDP vs. FOB Analysis
2026 freight volatility and customs complexity make Incoterms selection critical for cost predictability.
| Term | Cost Components Included | 2026 Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | Factory loading + ocean freight to port of discharge | High: Unpredictable customs clearance fees, port congestion surcharges (2026 avg. +18% YoY), inland transport risks | Large distributors with in-house logistics teams & bonded warehouses |
| DDP (Delivered Duty Paid) | Full door-to-door: freight, insurance, duties, taxes, last-mile delivery | Low: Single fixed price. Supplier bears compliance risk (requires trusted partner) | All clinics & new distributors – essential for budget certainty in 2026 |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a 19-year specialist in dental equipment export (est. 2007), Carejoy addresses critical 2026 sourcing challenges:
- Regulatory Assurance: Full ISO 13485:2016 certification (Scope: Dental Anesthetic Delivery Systems) + EU MDR-compliant CE marking under NB 2797. Technical files available for FDA/Health Canada submission support.
- MOQ Flexibility: Leverages 15,000m² Baoshan District factory for scalable production. Achieves competitive pricing at 5-unit MOQs through shared component platforms.
- DDP Expertise: Manages end-to-end logistics to 95+ countries with guaranteed landed cost quotes. Handles 2026-specific EPR/CDP compliance for EU shipments.
- Technical Integration: OEM/ODM capability for seamless integration with major practice management software (e.g., Dentrix, exocad).
2026 Recommendation: Request their “Anesthetic System DDP Cost Calculator” tool for real-time landed cost projections in your target market.
Shanghai Carejoy Medical Co., LTD – Direct Sourcing Channel
Core Competency: Factory-Direct Supply of Dental Anesthetic Delivery Systems (OEM/ODM Enabled)
Verification Required: Request Certificate # CN-2026-0887 (ISO 13485) & CE Certificate # MDR-CH-2026-ANES
Contact:
📧 [email protected]
💬 WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
🌐 Factory Audit: Available by appointment (Baoshan District, Shanghai)
Note: All 2026 quotes require submission of target market regulatory requirements for validation.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Top 5 FAQs When Purchasing a Dental Injection Machine – For Dental Clinics & Distributors
For technical specifications and procurement support, contact your authorized dental technology provider.
Need a Quote for Dental Injection Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


