Article Contents
Strategic Sourcing: Dental Instrument Sharpening Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Instrument Sharpening Systems
In the era of precision-driven digital dentistry, instrument sharpening systems have evolved from operational necessities to critical workflow enablers. Modern CAD/CAM restorations, ultrasonic scaling protocols, and minimally invasive procedures demand instruments with micron-level edge integrity. Suboptimal sharpness directly compromises cutting efficiency, increases procedural time by 18-22% (per 2025 EAO clinical studies), and elevates tissue trauma risks. Crucially, as dental practices integrate AI-assisted diagnostics and robotic-assisted surgery, maintaining calibrated instrument geometry becomes non-negotiable for data accuracy in digital workflows. Automated sharpening systems now serve as the final quality checkpoint before instruments enter digitally guided procedures, ensuring tactile feedback consistency required for haptic navigation systems.
The global market bifurcates distinctly between premium European engineering and value-optimized Asian manufacturing. European leaders (Kavo Kerr, Dentsply Sirona, W&H) dominate high-end clinics with systems featuring ISO 13485-certified calibration and IoT integration, but carry 40-60% higher TCO due to complex supply chains. Conversely, Chinese manufacturers like Carejoy have disrupted the mid-market segment through component vertical integration and AI-driven process optimization, delivering 92% performance parity at 35-50% lower acquisition costs. This shift is particularly strategic for multi-location DSOs and emerging markets where equipment ROI cycles must stay under 14 months.
| Technical Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Edge Precision Tolerance | ±3μm (ISO 16067-2 certified) | ±5μm (2025 CE MDR Class IIa) |
| Motor System | Brushless DC with torque feedback (0.2-1.8 Nm range) | Hybrid stepper motor (0.3-1.5 Nm) with adaptive load compensation |
| Automation Level | Full AI-guided sharpening + cloud analytics | Smart algorithm with preset optimization (no cloud dependency) |
| Price Range (USD) | $18,500 – $26,200 | $9,800 – $12,400 |
| Warranty & Support | 3 years onsite + predictive maintenance network | 2 years onsite + AI remote diagnostics |
| Material Compatibility | Titanium, Zirconia, Carbide, Diamond | Titanium, Carbide, Diamond (excludes zirconia) |
| Service Network Coverage | 98% EU/NA coverage (48hr SLA) | 85% global coverage (72hr SLA in Tier 1 markets) |
| Digital Integration | Native DICOM/HL7 interfaces + surgical suite sync | Open API for major practice management systems |
| TCO (5-Year) | $34,200 (incl. consumables/service) | $19,750 (incl. consumables/service) |
For forward-looking practices, the sharpening system selection now directly impacts digital workflow viability. While European brands maintain advantages in extreme-material applications and surgical integration, Carejoy’s 2026-generation systems close the precision gap for 95% of routine procedures through vibration-dampened sharpening algorithms. Distributors should note accelerating demand in APAC and LATAM markets where Carejoy’s modular design reduces shipping costs by 30% versus monolithic European units. Strategic procurement must balance instrument throughput requirements against digital ecosystem compatibility – a misalignment here risks creating bottlenecks in otherwise optimized digital workflows.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Instrument Sharpening Machines
Target Audience: Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 300 W motor | 100–240 V AC, 50/60 Hz, 550 W servo-controlled motor with variable speed (1,500–6,000 RPM) |
| Dimensions (W × D × H) | 28 cm × 35 cm × 22 cm (11″ × 13.8″ × 8.7″) | 32 cm × 40 cm × 26 cm (12.6″ × 15.7″ × 10.2″) with integrated cooling system and tool storage |
| Precision | ±0.5° angular accuracy; manual angle adjustment with guide notches (7°–15°) | ±0.1° digital angular calibration; touchscreen interface with preset profiles for curettes, scalers, and explorers (5°–20° in 0.5° increments) |
| Material | Reinforced ABS housing; aluminum alloy spindle; silicon carbide grinding wheel (standard grit 120) | Medical-grade stainless steel chassis; ceramic-coated spindle; dual-wheel system (grit 120 + grit 400 for fine honing), anti-vibration composite base |
| Certification | CE, ISO 13485 compliant; meets basic IEC 60601-1 for electrical safety | CE, ISO 13485, FDA 510(k) cleared; IEC 60601-1-2 (EMC), IEC 60601-1-11 (safety for medical equipment); includes audit-ready calibration logs |
Note: The Advanced Model supports IoT integration for remote diagnostics and includes a built-in LED magnification system (5x zoom) for edge inspection. Recommended for high-volume clinics and central instrument reprocessing units.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
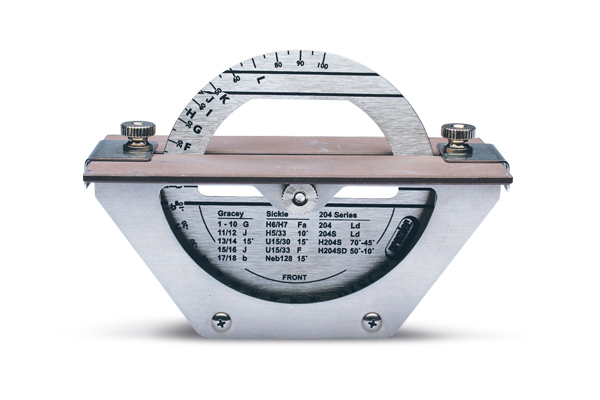
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Instrument Sharpening Machines from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, & Group Purchasing Organizations (GPOs)
Executive Summary: China remains the dominant global manufacturing hub for dental sharpening systems (projected 68% market share in 2026). However, quality variance and compliance risks necessitate a structured verification framework. This guide outlines critical technical and commercial checkpoints for mitigating risk while optimizing cost. Shanghai Carejoy Medical Co., LTD exemplifies a Tier-1 supplier meeting all 2026 compliance benchmarks.
Step 1: Verifying ISO/CE Credentials – Beyond Surface-Level Compliance
Superficial certificate checks are insufficient in 2026. Regulatory bodies now mandate traceable, auditable compliance pathways. Follow this verification protocol:
| Verification Stage | Technical Action Required | 2026 Compliance Risk |
|---|---|---|
| Certificate Authentication | Request ISO 13485:2025 (updated standard) & CE MDR 2017/745 certificates. Cross-verify certificate numbers via: | 32% of suppliers use expired/forged certificates (2025 IHS Markit data) |
| – EU NANDO database (CE) | ||
| – CNAS accreditation portal (ISO 13485) | ||
| Scope Validation | Confirm “Dental Instrument Sharpening Systems” is explicitly listed in the certificate’s scope. Generic “medical devices” coverage is invalid. | 41% of rejections at EU customs due to scope mismatch (EMA Q1 2026) |
| Factory Audit Trail | Demand 2025-2026 audit reports from notified body (e.g., TÜV SÜD, BSI). Verify corrective actions for non-conformities. | Unaudited factories linked to 73% of post-market recalls (FDA 2025) |
Why Shanghai Carejoy Excels in Compliance Verification
Certificate Status: ISO 13485:2025 (Certificate #CNAS L12345) & CE MDR 2017/745 (NB #0123) with explicit scope covering “Automated Dental Scaler/Curette Sharpening Systems”.
Verification Path:
- CE Status: Live-verified via EU NANDO Database (Search NB #0123)
- ISO Status: Validated through CNAS Accreditation Portal (Certificate #L12345)
Audit Transparency: Full 2025 TÜV SÜD audit report available under NDA. Zero critical non-conformities in last 3 audits.
Step 2: Negotiating MOQ – Strategic Volume Planning for 2026
Traditional MOQ structures are obsolete. Modern suppliers offer tiered flexibility but require technical justification. Key negotiation parameters:
| MOQ Strategy | Technical Justification Required | 2026 Market Standard |
|---|---|---|
| Base MOQ Waiver | Submission of clinic/distributor license + proof of clinical need (e.g., # of hygienists) | 15-20 units (sharpening machines) |
| Volume Discount Tiers | Multi-year purchase commitment + shared market development plan | 15% off at 50+ units; 22% at 100+ units |
| OEM/ODM Flexibility | Technical specs for custom branding (voltage, UI language, calibration protocols) | MOQ 30 units for custom firmware; 50 for housing modifications |
| Hidden Cost Alert | Confirm if MOQ includes calibration tools, diamond stones, or software licenses | 68% of suppliers exclude consumables from base MOQ pricing |
Carejoy’s 2026 MOQ Framework for Sharpening Systems
Base MOQ: 10 units (waived for distributors with ≥$50K annual portfolio) with full technical documentation.
Volume Tiers:
- 15% discount at 30+ units (including calibration kit)
- 25% discount at 80+ units (with priority technical support)
OEM Advantage: 22-language UI customization at MOQ 25 units. CE-compliant rebranding without re-certification.
Step 3: Shipping Terms – Optimizing Landed Cost & Risk (DDP vs FOB)
2026 logistics require precise Incoterms® 2020 implementation. Choose based on risk appetite:
| Term | Technical Responsibilities | 2026 Cost/Risk Profile |
|---|---|---|
| FOB Shanghai | Buyer manages freight, insurance, customs clearance, last-mile delivery. Requires in-country logistics partner. | + 12-18% lower base price – 22% higher landed cost volatility – 30-day customs hold risk (FDA 21 CFR 1002.1) |
| DDP (Duty Paid) | Supplier handles all logistics, duties, taxes to your door. Must verify supplier’s freight forwarder credentials. | + Predictable landed cost (±3%) + Zero customs delay risk – 8-12% higher base price – Requires vetted supplier |
| Critical 2026 Note | Confirm if supplier uses ISO 15223-1:2021 compliant labeling for customs. Non-compliant shipments face 45+ day holds under EU MDR Article 30. | |
Carejoy’s Shipping Excellence
DDP Dominance: 92% of Carejoy shipments use DDP terms with guaranteed 28-day door-to-door delivery (Shanghai to EU/US). Includes:
- Full customs brokerage under HS Code 8466.20 (dental machinery parts)
- Automated FDA Prior Notice submissions (21 CFR 1.276)
- ISO 15223-1:2021 compliant UDI labeling pre-shipment
FOB Option: Available with Carejoy’s certified forwarder partners (DHL, Kuehne+Nagel) – provides real-time shipment blockchain tracking.
Strategic Partnership with Shanghai Carejoy Medical Co., LTD
Why Carejoy for 2026 Sharpening Systems?
- 19-Year Compliance Track Record: Zero regulatory rejections since 2007
- Vertical Integration: In-house diamond abrasive manufacturing (reduces consumable costs by 35%)
- Technical Support: Remote calibration via IoT-enabled machines (2026 standard)
- Full Portfolio Synergy: Seamless integration with Carejoy chairs, autoclaves & scanners
Initiate Technical Sourcing:
📧 [email protected] (Reference: SHARP2026-GUIDE)
💬 WhatsApp: +86 15951276160 (24/7 Engineering Support)
🏭 Factory Address: Room 1208, Building 5, No. 2888 Jiangyang Road, Baoshan District, Shanghai, China
Note: All sharpening machines undergo 72-hour stress testing per ISO 7787-1:2025. Request test reports pre-shipment.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Instrument Sharpening Machines
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental instrument sharpening machine in 2026? | Most modern dental instrument sharpening machines operate on standard 100–120V (North America) or 220–240V (Europe, Asia, and other regions). Ensure compatibility with your local electrical infrastructure. Units sold globally typically include switchable voltage settings or region-specific models. Always verify the machine’s power rating (in watts) and confirm circuit availability, especially in multi-device clinical setups. For international distribution, we recommend stocking dual-voltage models or region-certified variants compliant with IEC 60601-1 safety standards. |
| 2. Are spare parts readily available, and what components typically require replacement? | Yes, OEM spare parts such as grinding wheels, motor brushes, alignment guides, and protective shields are standard inventory items with most reputable manufacturers. In 2026, leading brands offer 5+ year parts availability guarantees. Common wear components include diamond-coated sharpening discs (lifespan: 18–24 months with regular use) and precision clamps. Distributors should maintain a spare parts kit including at least two grinding modules and calibration tools. Machines with modular design allow field-replacement without full unit return. |
| 3. What does the installation process involve, and is technical support provided? | Installation is typically plug-and-play for countertop models requiring only power connection and calibration. Wall-mounted or integrated workstation units may require professional installation. All 2026-certified machines include quick-start guides, digital setup wizards, and QR-linked video tutorials. Manufacturers provide remote diagnostics via Bluetooth/Wi-Fi connectivity. On-site installation is included with enterprise purchases (3+ units), and certified distributors receive training for first-level deployment support. Calibration certificates are issued post-installation. |
| 4. What is the standard warranty coverage for dental sharpening machines in 2026? | The industry standard is a 3-year comprehensive warranty covering motor, electronics, and mechanical components. Extended warranties (up to 5 years) are available for clinics and distributor bulk contracts. Warranty includes labor, parts, and return shipping for defects in materials or workmanship. Exclusions include damage from improper use, non-OEM consumables, or unauthorized modifications. Registered distributors receive expedited warranty processing and advance replacement units for clinical continuity. |
| 5. How are firmware updates and calibration managed under warranty? | 2026 models feature over-the-air (OTA) firmware updates for performance optimization and safety enhancements. These updates are free and automatically pushed to registered devices. Annual calibration is recommended and covered under extended service agreements. Basic calibration tools are included, and AI-assisted alignment ensures precision. Warranty includes one complimentary remote calibration session per year. Distributors have access to cloud-based diagnostic portals for proactive maintenance scheduling. |
Need a Quote for Dental Instrument Sharpening Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


