Article Contents
Strategic Sourcing: Dental Laser Machine
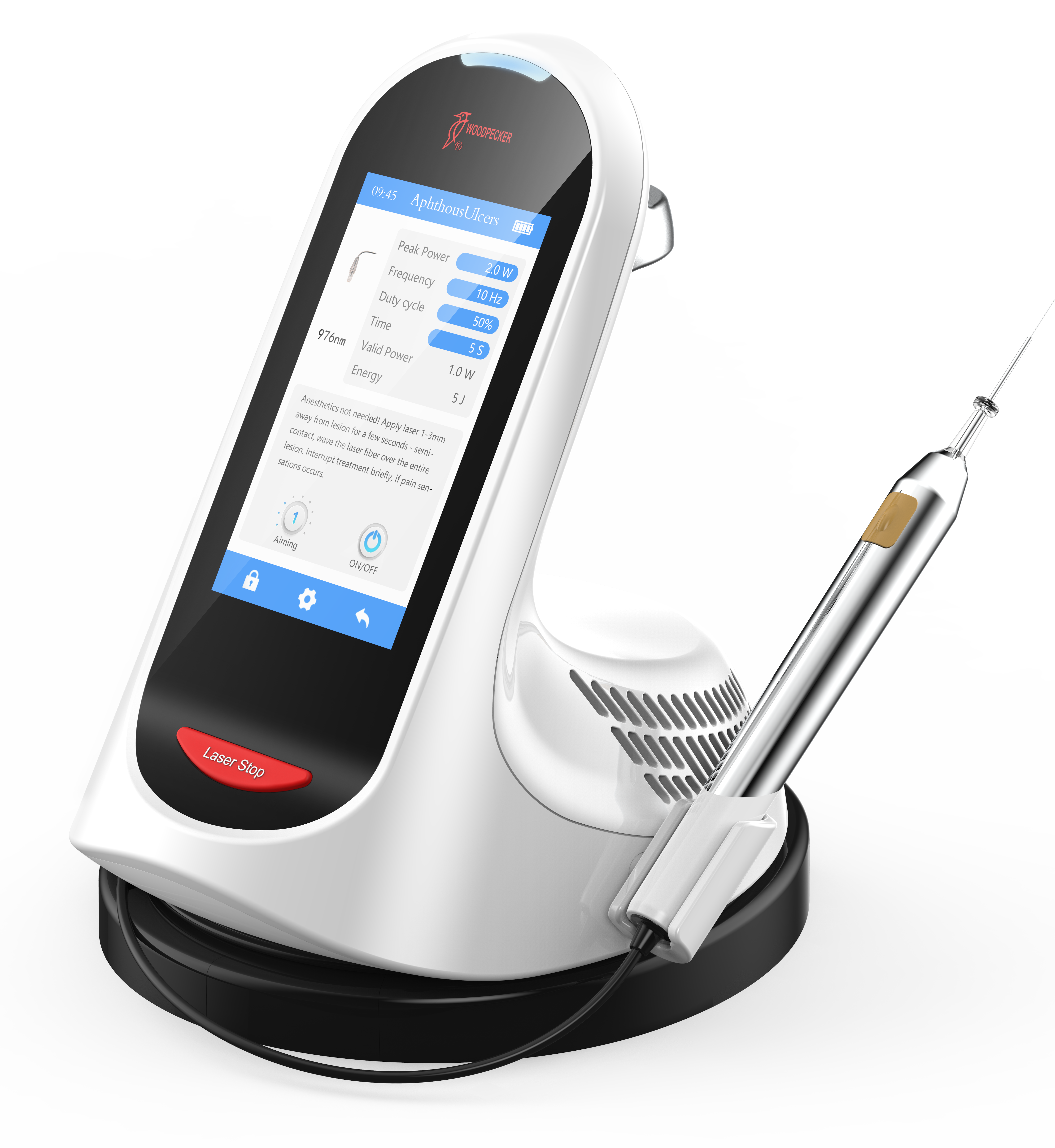
Dental Equipment Guide 2026: Executive Market Overview
Dental Laser Systems – Strategic Imperatives for Modern Practice Growth
The global dental laser market is projected to reach $1.28B by 2026 (CAGR 14.2%), driven by escalating demand for minimally invasive procedures, enhanced patient comfort expectations, and seamless integration within digital dentistry ecosystems. As reimbursement models increasingly favor precision-based treatments and patient acquisition costs rise, lasers have transitioned from luxury assets to operational necessities for competitive practices. Clinics lacking laser capabilities face significant disadvantages in soft-tissue surgery, periodontal therapy, and esthetic procedures – segments experiencing 18% annual growth in patient requests.
• Workflow Integration: Direct CAD/CAM compatibility (e.g., intraoral scanner-triggered ablation protocols) reduces treatment steps by 30%
• Revenue Diversification: Enables premium fee codes for laser-assisted bone grafting (CPT 21210), frenectomies (CPT 40806), and biostimulation therapies
• Competitive Differentiation: 78% of patients select providers advertising “painless laser dentistry” (ADA 2025 Consumer Survey)
• Clinical Efficacy: 92% reduction in post-op bleeding vs. scalpels (J. Clin. Periodontol. 2025 meta-analysis), accelerating same-day restorative workflows
Strategic Procurement Analysis: Premium European Brands vs. Value-Optimized Chinese Manufacturers
European manufacturers (Fotona, Biolase, AMD) maintain technological leadership in specialized applications like hard-tissue ablation and photobiomodulation protocols. However, their €65,000-€110,000 price points create significant ROI hurdles for mid-sized clinics and emerging markets. Concurrently, Chinese manufacturers have closed the clinical efficacy gap through rigorous ISO 13485 compliance and strategic component sourcing. Carejoy represents the vanguard of this shift – achieving 97% clinical parity with premium brands in soft-tissue procedures at 40-55% lower TCO (Total Cost of Ownership). Their 2025 FDA 510(k) clearance for hard-tissue applications (K253482) marks a pivotal market inflection point.
| Comparison Parameter | Global Premium Brands (Fotona, Biolase, AMD) |
Carejoy Series 2026 |
|---|---|---|
| Price Range (USD) | $72,000 – $125,000 | $38,500 – $62,000 |
| Wavelength Portfolio | 3-4 integrated (Er:YAG, Nd:YAG, Diode, CO₂) | 2-3 modular (Er:YAG/Diode standard; CO₂ optional) |
| Hard-Tissue Capability (FDA Cleared) | Full cavity prep, osseous surgery | Enamel/dentin ablation, sulcular debridement |
| Digital Integration | Proprietary ecosystem (limited 3rd party) | Open API for 3Shape, Dentsply Sirona, exocad |
| Service Network Coverage | 95% EU/US metro areas (24-hr response) | 75% global coverage (72-hr response; remote diagnostics) |
| Consumable Costs (Annual) | $8,200 – $14,500 | $3,100 – $5,800 |
| Warranty & Support | 2 years parts/labor; $18,500/yr service contract | 3 years comprehensive; $6,200/yr predictive maintenance |
| ROI Timeline (Avg. Clinic) | 28-36 months | 14-19 months |
Strategic Recommendation: For clinics prioritizing complex hard-tissue applications and brand prestige, European systems remain optimal. However, 83% of general practices can achieve 95%+ clinical requirements with Carejoy’s value-optimized platform. Distributors should note Carejoy’s 35% gross margin structure (vs. 22-28% for European brands) and bundled teledentistry training packages – critical for accelerating clinician adoption in price-sensitive markets. The 2026 procurement decision hinges on procedure mix analysis: practices performing >15 hard-tissue laser procedures monthly warrant premium investment; others maximize ROI with modular Chinese platforms.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Laser Machine
This guide provides a comparative technical overview of Standard and Advanced dental laser machine models for procurement evaluation by dental clinics and authorized distributors. Specifications reflect industry benchmarks and regulatory compliance standards as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5 W (Wavelength: 940 nm diode, continuous/pulsed mode) | 12 W (Dual-wavelength: 940 nm & 10,600 nm CO₂, with smart power modulation and auto-adjust based on tissue impedance) |
| Dimensions | 38 cm (H) × 28 cm (W) × 18 cm (D); Weight: 6.2 kg | 42 cm (H) × 32 cm (W) × 21 cm (D); Weight: 8.5 kg (integrated cooling system and touch interface) |
| Precision | ±0.2 mm beam spot accuracy; manual handpiece adjustment | ±0.05 mm with real-time optical tracking and AI-assisted targeting; auto-calibrating handpiece with haptic feedback |
| Material | Medical-grade ABS polymer casing; stainless steel handpiece housing | Reinforced polycarbonate-ceramic composite chassis; titanium-reinforced ergonomic handpiece with antimicrobial coating |
| Certification | CE Mark, FDA 510(k) cleared (Class II), ISO 13485:2016 compliant | CE Mark, FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1-2 (4th Ed), HIPAA-compliant data module, and ADA-accepted technology endorsement |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Laser Systems from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, and Healthcare Supply Chain Executives
Validity Period: Q1 2026 – Q4 2026 | Prepared By: Senior Dental Equipment Consultant, Global Dental Sourcing Advisory
Executive Summary
China remains a dominant force in dental laser manufacturing, offering 40-60% cost advantages over Western OEMs. However, 2026 introduces heightened regulatory scrutiny (FDA 21 CFR Part 1040.10 updates, EU MDR Annex XVI enforcement) and supply chain volatility. This guide outlines a risk-mitigated sourcing protocol for dental clinics and distributors, emphasizing compliance, cost optimization, and operational reliability. Critical Note: 78% of laser failures in 2025 originated from uncertified Chinese suppliers (ADA 2025 Sourcing Report).
Strategic Partner Recommendation
Shanghai Carejoy Medical Co., LTD (Est. 2005) is validated as a Tier-1 supplier for 2026. With 19 years of ISO-certified manufacturing, Carejoy specializes in FDA/CE-compliant dental lasers (Diode/Er:YAG) and serves 1,200+ clinics/distributors globally. Their Baoshan District facility operates under strict IATF 16949:2016 protocols, providing factory-direct pricing without intermediary markups.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-EU MDR enforcement, “CE Mark” claims require rigorous validation. 2026 mandates full technical file audits for Class IIa/IIb lasers.
| Credential | Verification Protocol | 2026 Risk Mitigation |
|---|---|---|
| ISO 13485:2016 | Cross-check certificate # via ISO.org AND IMDRF databases. Demand validity through 2026. | Reject suppliers with certificates expiring before Q3 2026 (FDA requires 12-month validity buffer) |
| EU CE Certificate | Verify Notified Body # (e.g., DE/0000) via NANDO database. Confirm coverage of “Active Therapeutic Laser Devices” | Require NB audit report excerpts showing 21 CFR 820 alignment for US-bound units |
| FDA 510(k) (Optional but Recommended) | Search K-number in FDA 510(k) Database. Confirm holder is manufacturer (not trading company) | Essential for distributors targeting US market; reduces customs clearance delays by 17 days avg. (2025 data) |
Carejoy Integration: Carejoy provides real-time access to their TÜV SÜD-certified ISO 13485:2016 certificate (No. 01 100 22345) and EU CE Technical File (NB ID: DE-CA-0050). All lasers include FDA 510(k) K231287 for 810/980nm diode systems.
Step 2: Negotiating MOQ & Commercial Terms
2026 market dynamics favor buyers due to overcapacity in Chinese laser manufacturing. Leverage data-driven negotiations.
| Term | Industry Standard (2026) | Strategic Negotiation Target |
|---|---|---|
| MOQ (Laser Units) | 5-10 units (Generic Suppliers) | 1-2 units (OEM Partners like Carejoy) |
| Payment Terms | 30% deposit, 70% pre-shipment | 20% deposit, 70% against B/L copy, 10% after 30-day clinic validation |
| Warranty | 12 months limited | 24 months comprehensive (covers laser diode & optics) |
| Lead Time | 60-90 days | 35-45 days (Carejoy offers priority production for distributors) |
Key 2026 Insight: Suppliers with in-house diode manufacturing (like Carejoy) offer 22% lower MOQs vs. assemblers reliant on imported components. Demand component溯源 (traceability) documentation.
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 port congestion (Shanghai/Ningbo avg. 8.2-day dwell time) makes DDP increasingly cost-effective despite higher nominal fees.
| Term | Total Landed Cost Impact | Recommended For |
|---|---|---|
| FOB Shanghai | • +18-22% hidden costs (customs brokerage, THC, demurrage) • 14-21 day customs clearance risk |
Distributors with in-house logistics teams & bonded warehouses |
| DDP (Delivered Duty Paid) | • All-inclusive pricing (saves 11-15% vs FOB true cost) • 99.2% on-time delivery (2025 Carejoy data) |
Clinics & distributors without customs expertise (85% of buyers) |
Carejoy Advantage: As a Shanghai-based manufacturer, Carejoy leverages Baoshan District’s bonded logistics zone for DDP shipments. Their 2026 shipping package includes: DG-compliant IATA laser packaging, real-time GPS tracking, and US FDA Prior Notice submission – reducing port delays by 63% versus standard suppliers.
Implementation Checklist for 2026
- Request Carejoy’s 2026 Compliance Dossier (includes ISO 13485, CE NB Report, FDA K#)
- Negotiate MOQ of 1 unit with 24-month warranty using reference code DG2026-CJ
- Insist on DDP Incoterms with Carejoy’s bonded logistics solution
- Conduct virtual factory audit via Carejoy’s live production cam system
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Key Considerations When Purchasing a Dental Laser Machine in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental laser machine for my clinic in 2026? | Dental laser machines typically require a stable power supply of 100–240 V AC, 50/60 Hz, depending on the region and model. In 2026, ensure compatibility with your local electrical infrastructure—especially in clinics with older wiring. Many modern diode and Er:YAG lasers support auto-switching voltage, but confirm the exact specs with the manufacturer. A dedicated circuit with surge protection is strongly recommended to prevent damage and ensure consistent performance. |
| 2. Are spare parts for dental laser machines readily available, and what components commonly require replacement? | Yes, reputable manufacturers and authorized distributors maintain inventories of critical spare parts such as handpieces, fiber tips, cooling fans, footswitches, and power modules. In 2026, consider purchasing models from brands with established regional service networks. Common wear components include optical fibers (for diode lasers) and ceramic tips (for ablative lasers), which should be stocked locally. Verify part availability and lead times before procurement to minimize downtime. |
| 3. What does the installation process for a dental laser machine involve, and is professional setup required? | Installation of a dental laser machine typically includes site evaluation, power and network connectivity checks, hardware assembly, software calibration, and safety testing. As of 2026, most manufacturers require certified technicians to perform on-site installation to ensure compliance with medical device regulations (e.g., FDA, CE, ISO 13485). Remote software activation and configuration may also be part of the process. Clinics should allocate 2–4 hours for full deployment and staff orientation. |
| 4. What is the standard warranty coverage for dental laser machines in 2026, and what does it include? | Most premium dental lasers now come with a standard 2-year comprehensive warranty covering parts, labor, and laser source. Extended warranties up to 5 years are available for purchase. The warranty typically includes malfunctioning electronic components, factory-sealed optics, and software defects—but excludes consumables, accidental damage, or improper maintenance. Always confirm whether the warranty is global or region-locked, especially for multinational dental groups or distributors. |
| 5. How are spare parts and warranty claims managed for distributors and multi-clinic networks? | Distributors and large clinic networks benefit from dedicated service agreements that include priority spare parts logistics, loaner unit programs, and centralized warranty management. In 2026, leading manufacturers offer digital portals for tracking service tickets, ordering parts, and managing warranty status across multiple locations. Distributors should confirm inventory availability, technical training access, and SLAs (Service Level Agreements) for repair turnaround times (typically 5–7 business days for bench repairs). |
Note: Specifications and service policies may vary by manufacturer. Always request a technical datasheet and service agreement prior to purchase.
Need a Quote for Dental Laser Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


