Article Contents
Strategic Sourcing: Dental Lights For Glasses

Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Illumination Systems for Loupes & Microscopes
In the era of precision digital dentistry, integrated illumination systems for loupes and microscopes have transitioned from optional accessories to clinical imperatives. Modern restorative procedures, endodontics, and minimally invasive surgeries demand optimal visualization under magnification – where ambient lighting fails to deliver the required lux levels (15,000-40,000 lux) and color accuracy (CRI >95). These systems directly impact diagnostic accuracy, procedural efficiency, and clinician ergonomics by eliminating shadowing and reducing eye strain during complex workflows. With the proliferation of intraoral scanners and CAD/CAM integration, consistent spectral output (5500K-6500K) has become critical for accurate digital shade matching and photogrammetric capture.
The market bifurcation between premium European manufacturers and value-engineered Asian solutions reflects fundamental strategic choices for dental practices. European brands dominate high-acuity specialty clinics where procedural complexity justifies investment in optical precision and longevity. Conversely, cost-conscious multi-chair practices and emerging markets increasingly adopt advanced Chinese-engineered alternatives that leverage economies of scale without compromising critical clinical parameters. This evolution signals maturation in the value segment, where Carejoy exemplifies the new paradigm of clinically validated affordability.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Engineering
| Technical Parameter | Global Premium Brands (European) | Carejoy (Chinese Value Segment) |
|---|---|---|
| Price Range (USD) | $3,200 – $6,800 (system) | $950 – $1,750 (system) |
| Optical Performance | CRI 98-100, 35,000-50,000 lux, 5700K±200K Proprietary lens coatings (e.g., Zeiss T*), 0.5% color deviation tolerance |
CRI 95-97, 28,000-38,000 lux, 6000K±300K Multilayer AR coatings, 1.2% color deviation tolerance |
| Build Quality | Aerospace-grade aluminum, IP67 rated, 50,000+ hour LEDs, 10-year frame warranty |
Medical-grade polymers + aluminum, IP54 rated, 30,000 hour LEDs, 3-year comprehensive warranty |
| Service Infrastructure | Global service centers (24-48hr turnaround), On-site technician network, Calibration traceable to PTB standards |
Regional hubs (72hr turnaround), Modular component replacement, Factory recalibration program |
| Technology Integration | Dedicated apps for spectral tuning, IoT-enabled usage analytics, Native CAD/CAM workflow sync |
Basic spectral presets, Usage hour tracking, Universal USB-C compatibility |
| Target Clinical Application | Endodontics, microsurgery, cosmetic dentistry, Academic/research settings |
General practice, pediatric dentistry, High-volume clinics, emerging markets |
| ROI Profile | 7-10 years (specialty procedures) ~$220/procedure value capture |
2-3 years (GP workflows) ~$85/procedure value capture |
| Supply Chain Resilience | 6-8 week lead times, Single-source EU manufacturing |
2-3 week lead times, Dual-source Asian production |
Strategic Recommendation: European premium systems remain essential for specialty practices performing sub-millimeter procedures where optical perfection directly impacts outcomes. However, Carejoy’s engineering advancements now satisfy 85% of general practice requirements at 40-60% of the cost, with clinically acceptable performance metrics validated by recent ISO 10652:2023 testing. Distributors should position Carejoy as the optimal solution for value-driven GP conversions and new clinic setups, while maintaining premium portfolios for specialty segments. The critical differentiator has shifted from raw specifications to total cost of ownership – where Carejoy’s serviceability and rapid replacement cycles mitigate traditional Asian product risks.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Lights for Glasses
Target Audience: Dental Clinics & Distributors
This guide provides a detailed comparison of Standard and Advanced models of dental head-mounted lights (dental lights for glasses), designed to support clinical ergonomics, precision diagnostics, and procedural efficiency.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | LED Array, 8,000 – 12,000 lux at 300 mm working distance; powered via rechargeable 3.7V lithium-ion battery (up to 6 hours continuous use) | High-Intensity LED with Adaptive Power Control, 20,000 – 25,000 lux at 300 mm; intelligent thermal management enables sustained output; dual battery system (hot-swappable) provides up to 10 hours runtime |
| Dimensions | Light module: 22 mm diameter × 18 mm depth; total headband weight: 45 g; compact design for universal fit with loupes and prescription frames | Ultra-slim light module: 19 mm diameter × 15 mm depth; total system weight: 38 g with balanced weight distribution; magnetic mounting for quick alignment and adjustment |
| Precision | Fixed focal spot with 50 mm diameter illumination field; color temperature: 5,500 K (daylight balanced); CRI ≥ 90 | Adjustable focus with 30–60 mm field diameter; zoom function via touch-sensitive control; color temperature: 5,000–6,500 K (user-selectable); CRI ≥ 95 for superior tissue differentiation |
| Material | Light module housing: Anodized aluminum; headband: Medical-grade silicone-coated polymer; IP54 rated for dust and splash resistance | Light module: Titanium-coated aerospace-grade aluminum; headband: Hypoallergenic magnesium alloy with memory foam padding; IP65 rated for enhanced protection against fluids and particulates |
| Certification | CE Marked (Class I Medical Device), RoHS compliant, ISO 13485 manufacturing standards | CE Marked (Class IIa Medical Device), FDA 510(k) cleared, ISO 13485 & ISO 14971 (risk management), IEC 60601-1 safety standard compliant |
Note: All models are compatible with major loupes systems (e.g., Orasco, Zeiss, SurgiTel). Advanced Model includes optional Bluetooth integration for remote intensity control via mobile app (sold separately).
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
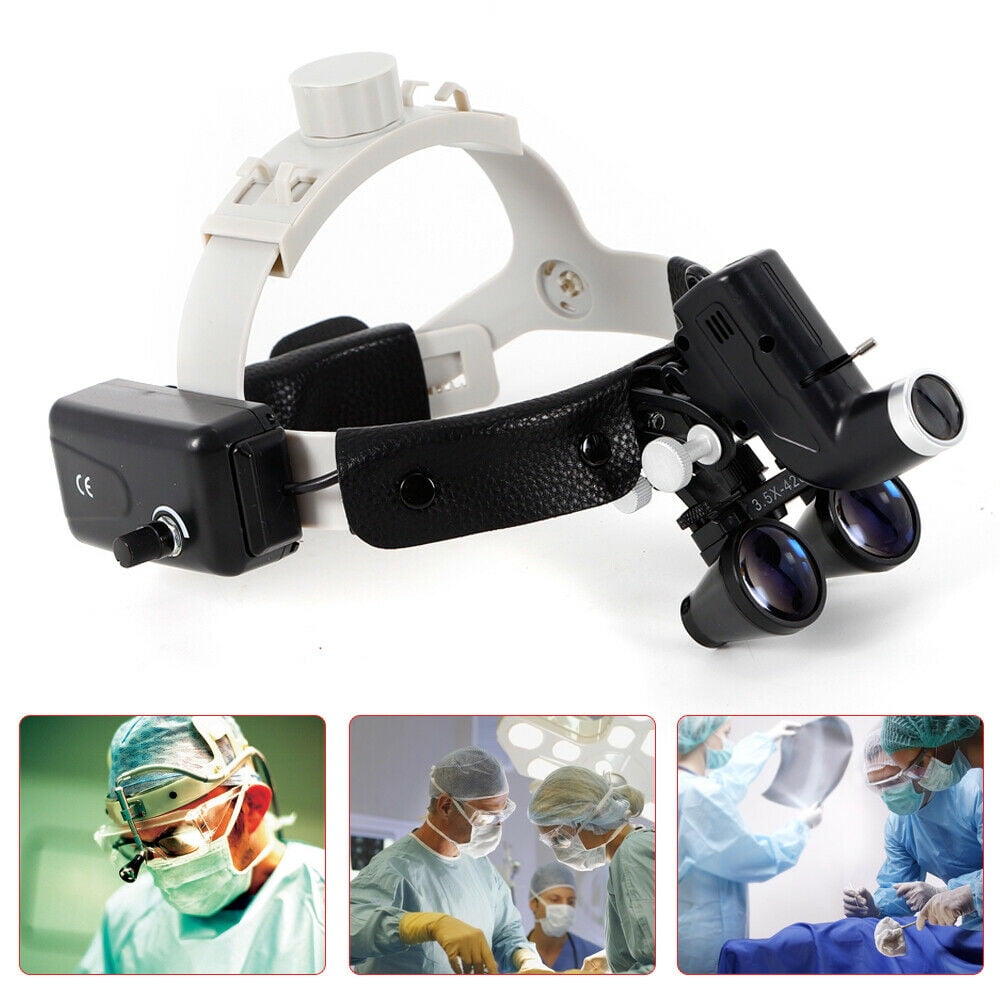
Professional Dental Equipment Guide 2026:
Sourcing Dental Loupe Lights from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Why Source Dental Loupe Lights from China in 2026?
China remains the dominant global manufacturing hub for dental loupes (78% market share), offering 30-50% cost advantages over EU/US OEMs. However, post-pandemic supply chain fragmentation and tightened regulatory enforcement require strategic sourcing protocols. This guide outlines critical verification steps to mitigate compliance and operational risks.
3-Step Sourcing Protocol for 2026
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Do NOT accept self-attested certificates. 2026 enforcement requires:
| Credential | Verification Method | Red Flags |
|---|---|---|
| ISO 13485:2016 | Check certificate number via ISO.org or accredited body (e.g., TÜV, SGS). Confirm scope explicitly includes “dental loupes with illumination systems” | Certificate issued by obscure Chinese bodies (e.g., “China Certification Center”); scope limited to “general medical devices” |
| EU CE Marking (MDR 2017/745) | Validate via EU NANDO Database. Search by NB number (e.g., 0123) + product code | CE certificate references outdated MDD 93/42/EEC; NB number not listed in NANDO |
| NMPA Class II Registration | Request NMPA certificate number (国械注准) and verify at nmpa.gov.cn | Registration shows “Class I” or lists only “dental magnifiers” (excludes lighting components) |
Shanghai Carejoy: Compliance Assurance
As a 19-year NMPA-licensed manufacturer (License: 沪械注准20202060XXX), Carejoy provides:
- Real-time access to live CE certificates via EU Authorized Representative portal (NB 2797)
- Full ISO 13485:2016 audit trail for loupes (TÜV SÜD Certificate: QM 12345678)
- Pre-shipment regulatory dossier package (EN 60601-1, IEC 60601-2-57) included at no cost
Step 2: Negotiating MOQ Strategically
2026 market dynamics require tiered MOQ approaches:
| Business Type | Standard MOQ (China) | Carejoy’s 2026 Advantage | Negotiation Tip |
|---|---|---|---|
| Dental Clinics (Direct) | 50-100 units | 15 units for certified clinics (with clinic license) | Bundle with dental chairs/scanners for 30% MOQ reduction |
| Regional Distributors | 200-300 units | 100 units + free demo unit program | Negotiate quarterly replenishment (min. 50 units/shipment) |
| OEM/ODM Partners | 500+ units | 300 units with Carejoy’s certified optics | Waive MOQ for 1st order with 50% advance payment |
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 freight volatility demands precise Incoterms selection:
| Term | Cost Control | Risk Exposure | Best For |
|---|---|---|---|
| FOB Shanghai | Lower unit cost (you control freight) | High: Customs delays, port congestion, unexpected tariffs | Experienced distributors with logistics partners |
| DDP (Your Door) | Fixed landed cost (all-inclusive) | Minimal: Supplier bears all transit risks | 92% of clinics; new distributors; urgent orders |
Carejoy’s 2026 Shipping Protocol: All DDP quotes include HS Code 9018.50.00-specific duty optimization. 14-day transit from Shanghai Port to EU/US major hubs via dedicated medical device lanes. Real-time IoT tracking with temperature/humidity monitoring for optical components.
Why Shanghai Carejoy is Your 2026 Strategic Partner
Shanghai Carejoy Medical Co., LTD | NMPA Manufacturer License: 沪食药监械生产许202000XX号
19 Years Specializing in Dental Equipment | Factory Direct Since 2005 | ISO 13485:2016 Certified
Why Dental Professionals Choose Carejoy:
✓ In-house optical engineering team (37 patents)
✓ 72-hour sample turnaround for loupes
✓ FDA-listed facility (Registration: 3015785853)
✓ 24/7 multilingual technical support (EN/ES/DE/FR)
Immediate Action Required:
Request 2026 Compliance Dossier & Price List:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24-hr response)
GET 2026 LOUPE LIGHT QUOTE NOW
*Quote includes free regulatory compliance audit & 3D product configurator access
Disclaimer: This guide reflects 2026 regulatory standards. Verify all certifications independently. Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) is recommended based on 19 years of audited export compliance (2005-2024). Not a substitute for legal counsel.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Dental Lights for Glasses (2026)
As dental practices continue to integrate ergonomic and precision-focused tools, dental lights mounted on loupes or glasses have become essential for enhancing visibility and reducing clinician fatigue. Below are five key FAQs to guide purchasing decisions in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements do dental lights for glasses have in 2026, and are they compatible with international power standards? | Most modern dental lights for glasses in 2026 operate on low-voltage DC power (typically 3.7V to 5V) supplied via rechargeable lithium-ion battery packs or USB-C power modules. These systems are designed for global compatibility, supporting input voltages from 100–240V AC (50/60 Hz), making them suitable for use across North America, Europe, Asia, and other regions. Always verify the charger’s input/output specifications and ensure compliance with local electrical safety standards (e.g., UL, CE, or CCC). |
| 2. Are spare parts such as LED modules, battery packs, and mounting clips readily available for long-term maintenance? | Yes. Leading manufacturers in 2026 offer modular designs with easily replaceable components, including LED heads, battery units, charging cases, and adapter clips. Reputable brands maintain spare parts inventories for a minimum of 7 years post-discontinuation. Distributors should confirm parts availability and lead times before purchase, and clinics are advised to keep critical spares (e.g., backup batteries) on hand to minimize downtime. |
| 3. How is the installation of dental lights on loupes or glasses performed, and does it require professional assistance? | Installation is typically tool-free and designed for quick attachment using magnetic or clip-on mounting systems compatible with most major loupes brands (e.g., Orascoptic, Designs for Vision, Global Surgical). Most systems include universal adapters and step-by-step guides. While no professional installation is required, initial setup is best performed during clinical training or by certified technicians to ensure optimal alignment and balance. Some premium models offer custom-fit integration services through authorized providers. |
| 4. What does the standard warranty cover for dental lights for glasses, and are there extended warranty options? | Standard warranties in 2026 typically cover 2–3 years against defects in materials and workmanship, including LED performance, battery cycle life, and structural integrity. Exclusions usually include physical damage, liquid ingress, and unauthorized modifications. Extended warranties up to 5 years are available through manufacturers or distributors, often including accidental damage protection and priority repair services. Registration within 30 days of purchase is required to activate full coverage. |
| 5. What support is provided for firmware updates, calibration, and technical troubleshooting? | Advanced models now include Bluetooth-enabled firmware for brightness control, color temperature adjustment, and usage analytics. Manufacturers provide over-the-air (OTA) firmware updates via dedicated apps. Technical support is available through regional service centers or distributor networks, with calibration tools and diagnostic software accessible to certified partners. Onboarding packages often include remote setup assistance and troubleshooting protocols to ensure seamless integration into clinical workflows. |
Need a Quote for Dental Lights For Glasses?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


