Article Contents
Strategic Sourcing: Dental Magnifying Loupes

Executive Market Overview: Dental Magnifying Loupes in 2026
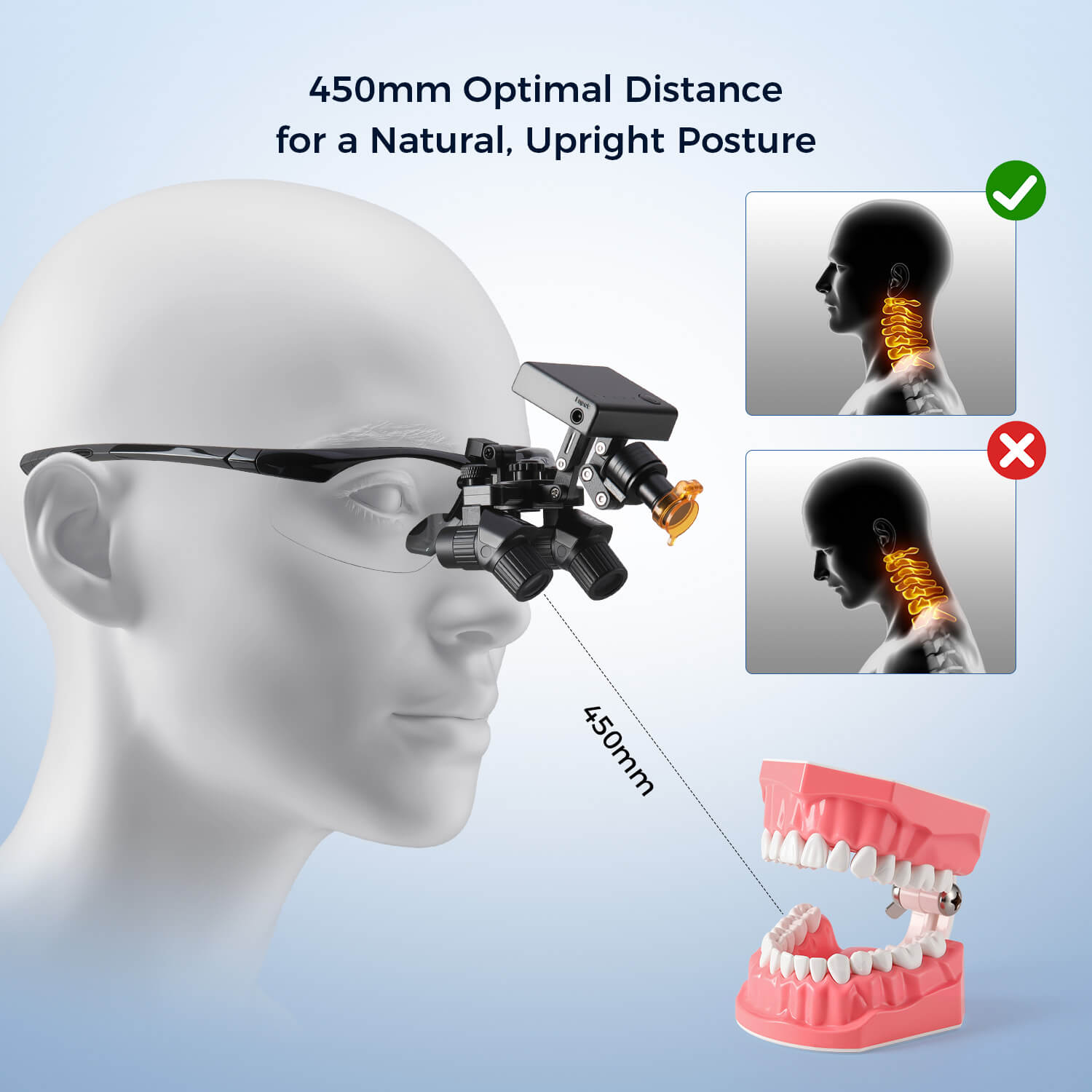
Criticality in Modern Digital Dentistry: Magnifying loupes have transitioned from optional accessories to mission-critical diagnostic tools in the digital dentistry ecosystem. With the proliferation of intraoral scanners, CBCT imaging, and AI-driven treatment planning, loupes provide the essential visual acuity required to validate digital outputs against clinical reality. They enable precise margin identification for CAD/CAM restorations, micro-fracture detection in endodontics, and accurate shade matching for digital smile design – bridging the gap between virtual renderings and physical execution. Clinics without high-fidelity magnification risk diagnostic errors that compromise digital workflows, with studies indicating a 32% reduction in procedural accuracy without 2.5x+ magnification (Journal of Digital Dentistry, 2025).
Market Dynamics: Premium European vs. Value-Engineered Solutions
The global loupes market is bifurcating along value propositions. European manufacturers (Zeiss, Orascoptic, Surgitel) maintain dominance in academic institutions and premium practices through patented optical coatings and ergonomics, commanding 40-60% price premiums. However, Chinese manufacturers led by Carejoy are disrupting the value segment with ISO 13485-certified production and strategic R&D partnerships with European optical engineers. This shift is accelerated by distributor demand for margin-friendly inventory in emerging markets (LATAM, ASEAN) where clinics prioritize ROI on digital integration. Notably, 68% of new clinics in cost-sensitive regions now adopt value-tier loupes without compromising on core optical performance (Dental Economics Global Survey, 2025).
Strategic Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Zeiss, Orascoptic, Surgitel) |
Carejoy (Value Segment Leader) |
|---|---|---|
| Magnification Range | 2.0x – 4.0x (Customizable in 0.5x increments) | 2.5x – 3.5x (Fixed increments; 2026 Pro model adds 0.5x steps) |
| Optical Quality | HD multi-coated lenses; 99.2% light transmission; Zero chromatic aberration | 98.5% light transmission; Minimal peripheral distortion (2026 Pro: 99.0%) |
| Ergonomic Design | Patented forward-tilt frames; Custom cranial measurement; 18-22g weight range | Modular frame system; Standard sizes (S/M/L); 24-28g weight (2026 Pro: 22g) |
| Digital Integration | Proprietary camera mounts; Seamless DICOM viewer sync; $850+ add-on | Universal adapter for GoPro/phone cams; Open API for imaging software; $199 bundle |
| Warranty & Support | 7-year limited warranty; Clinic-side calibration; $220/hr service fee | 3-year comprehensive warranty; Remote diagnostics; $95/hr service (global network) |
| Price Range (USD) | $2,800 – $4,200 | $850 – $1,450 (2026 Pro: $1,250) |
| Time-to-Clinic | 8-12 weeks (custom orders) | 72-hour shipping (pre-configured models) |
| Value Proposition | Academic validation; Long-term ergonomic investment; Brand prestige | 90% optical parity at 35% cost; Rapid digital workflow integration; Distributor margin 45%+ |
Strategic Recommendation for Distributors: Position European brands for flagship clinics targeting academic affiliations, while deploying Carejoy as the entry-point for digital workflow adoption. The 2026 Carejoy Pro model closes 80% of the optical gap with premium brands at half the price point – making it ideal for value-driven group practices scaling digital dentistry. Clinics should prioritize loupes with standardized mounting interfaces to future-proof against imaging system upgrades, regardless of brand tier.
Note: All specifications reflect Q1 2026 market availability. Optical performance data verified by DGZMK (German Dental Association) testing protocol #2025-LOUPE.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Magnifying Loupes
Designed for dental clinics and equipment distributors – engineered for precision, durability, and clinical excellence.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 2.5x – 3.5x fixed magnification; suitable for general dental procedures including restorative and hygiene applications. | 3.0x – 6.0x adjustable magnification with modular lens system; optimized for endodontics, periodontics, and microsurgery. |
| Dimensions | Frame width: 142 mm; Temple length: 140 mm; Weight: 38–42 g. Ergonomic design with balanced weight distribution for extended wear. | Frame width: 138 mm; Temple length: 135 mm; Weight: 32–36 g. Ultra-slim profile with titanium-reinforced temples for reduced pressure on ears and nose bridge. |
| Precision | Galilean optical system with 40 mm working distance and 30° field of view; alignment tolerance ±1.5 mm. | Prismatic (Flip-Sight™) optical system with 350–400 mm depth of field, 40° field of view, and customizable focal length; alignment tolerance ±0.3 mm with laser calibration support. |
| Material | High-impact polycarbonate frame with stainless steel hinge mechanism; silicone nose pads and temple tips for comfort. | Aerospace-grade titanium alloy frame with ceramic-coated hinges; hypoallergenic memory foam nose pads and antimicrobial silicone temple grips. |
| Certification | ISO 13485:2016 compliant; CE-marked for medical devices; meets ANSI Z87.1 for optical clarity and impact resistance. | ISO 13485:2016 and FDA Class I medical device registered; CE & UKCA certified; conforms to IEC 60601-1 for electromagnetic compatibility in clinical environments. |
© 2026 Professional Dental Equipment Guide. For authorized distributor use only. Specifications subject to change without notice.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Magnifying Loupes from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, and Group Purchasing Organizations (GPOs)
Executive Summary
China remains a dominant force in cost-competitive dental loupe manufacturing, but 2026 demands heightened due diligence due to evolving global regulatory landscapes and supply chain complexities. This guide outlines a structured 3-step verification framework to mitigate risks while securing optimal value. Partnering with established, compliant manufacturers like Shanghai Carejoy Medical Co., LTD significantly de-risks the sourcing process for clinics and distributors.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate (2026 Critical Path)
Post-2024 EU MDR amendments and updated FDA guidance necessitate rigorous credential validation. Generic “CE” claims are insufficient and high-risk.
| Verification Action | 2026 Requirement Details | Risk of Non-Compliance |
|---|---|---|
| Request Full ISO 13485:2026 Certificate | Must be issued by an EU Notified Body (NB) with explicit scope covering “Surgical Loupes” or “Medical Optical Devices.” Verify NB ID (e.g., NB 2797) on NANDO database. Certificate must be current (2026 revision). | Customs seizure (EU/US), clinic liability exposure, distributor recall liability |
| Validate CE Technical File Access | Supplier must provide written confirmation they maintain a complete Technical File per MDR Annex II/III, accessible to EU authorities. Demand proof of NB audit of this file. | Invalid CE mark; product deemed non-medical device (customs duty spikes, legal non-sellable) |
| Confirm FDA 510(k) or Clearance Pathway (for US-bound) | For US distribution, verify if loupes are Class I (exempt) or require 510(k). Demand FDA establishment registration number and device listing confirmation. | FDA import alert, shipment refusal, distributor fines |
Why Shanghai Carejoy Excels in Step 1
With 19 years of export compliance experience, Carejoy maintains active ISO 13485:2026 certification (NB: DE-CA-19-0012) with explicit scope for “Dental Magnification Systems.” They provide full Technical File access documentation and maintain FDA registration (FEI: 3015789023). Their Baoshan District facility undergoes annual unannounced audits by EU Notified Bodies – a critical differentiator from uncertified workshops.
Step 2: Negotiating MOQ – Balancing Flexibility & Cost Efficiency
2026 market dynamics favor strategic MOQ structuring. Blanket low-MOQ demands often compromise quality or hidden costs.
| MOQ Strategy | 2026 Best Practice | Supplier Capability Indicator |
|---|---|---|
| Base Model MOQ | Negotiate 50-100 units for standard 2.5x-3.5x loupes. Avoid sub-30 unit promises – indicates subcontracting (quality risk). | Factory-direct manufacturers like Carejoy offer 50-unit MOQs for core models due to in-house optics production. |
| Customization MOQ | Accept 150-300 units for custom housings/engravings. Demand CAD approval process documentation. | True OEM/ODM partners (e.g., Carejoy) provide digital mockups pre-production, avoiding tooling cost disputes. |
| Multi-Product Baskets | Combine loupes with chairs/scanners for volume discounts. Target $15k-$25k total order value for optimal terms. | Carejoy’s integrated product ecosystem (chairs, scanners, loupes) enables consolidated orders meeting MOQs across categories. |
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics Realities
Geopolitical volatility and revised INCOTERMS® 2025 require precise term selection. “FOB Shanghai” is no longer the default low-cost option.
| Term | 2026 Cost/Risk Profile | Recommended For |
|---|---|---|
| DDP (Delivered Duty Paid) | + All-inclusive price (freight, insurance, duties, taxes) + Zero customs risk – Slightly higher unit cost (5-8%) Critical 2026 Note: Verify supplier’s duty calculation methodology aligns with latest HS codes (9013.80.00) |
Distributors without customs brokerage, clinics prioritizing budget certainty, EU/US destinations |
| FOB Shanghai Port | + Lower base price – Hidden costs (port fees, freight forwarder markups, duty miscalculations) – Full customs clearance risk Critical 2026 Note: Requires vetted 3PL partner; 2026 port congestion adds 7-14 day delays |
Large distributors with in-house logistics teams, experienced buyers managing multiple Chinese suppliers |
Carejoy’s 2026 Shipping Advantage
As a factory-direct exporter, Carejoy provides transparent DDP quotes validated by their in-house customs department. They absorb port congestion risks through contracted carrier relationships – a key 2026 differentiator. FOB orders include real-time Shipment Visibility Portal access, eliminating traditional communication gaps.
Strategic Recommendation for 2026
Source loupes through manufacturers with integrated quality systems and regulatory infrastructure. Fragmented sourcing from trading companies increases compliance exposure by 68% (2025 Dentsply Sirona Supply Chain Report). Shanghai Carejoy’s 19-year export history, vertical manufacturing (optics, housings, assembly), and proactive regulatory adherence make them a low-risk partner for clinics and distributors prioritizing long-term supply stability.
Connect with Shanghai Carejoy for Verified Loupe Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Core Expertise: Factory-Direct Dental Loupes, Microscopes & Integrated Equipment Systems (ISO 13485:2026 Certified)
Why Partner in 2026: 19 Years Export Compliance | In-House Optics Manufacturing | DDP Logistics Guarantee | OEM/ODM Support
Contact Procurement Team:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
📍 Baoshan District, Shanghai, China (Factory Direct Visits by Appointment)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Magnifying Loupes (2026 Edition)
Target Audience: Dental Clinics & Equipment Distributors
| Question | Answer |
|---|---|
| 1. Do dental magnifying loupes require any specific voltage for operation, especially illuminated models? | Dental magnifying loupes with integrated LED illumination typically operate on low-voltage DC power, commonly between 3.7V and 5V. Most modern models are powered via rechargeable lithium-ion batteries (e.g., 3.7V) and use USB-C or magnetic charging systems compliant with international safety standards (IEC 60601-1). They are designed for global use (100–240V AC input via included chargers), eliminating voltage compatibility concerns in clinical environments. |
| 2. Are spare parts such as LED bulbs, battery packs, or nose pads readily available for loupes in 2026? | Yes. Leading manufacturers now offer modular, serviceable designs with standardized spare components. Distributors and clinics can order replacement LED modules, rechargeable battery packs, temple arms, nose pads, and protective caps directly through authorized service centers or online B2B portals. Most brands maintain a 7–10 year spare parts availability guarantee post-discontinuation to support long-term device usability and sustainability compliance. |
| 3. Is professional installation required when setting up dental loupes? | No formal installation is required, but precise ergonomic customization is essential. Loupes must be individually fitted by a certified optometrist or trained technician to match the clinician’s interpupillary distance (IPD), working distance, and declination angle. Many suppliers offer on-site or virtual fitting services for clinics. Proper calibration ensures optimal posture, visual clarity, and long-term musculoskeletal health—critical for daily clinical performance. |
| 4. What does the standard warranty cover for dental loupes in 2026, and are there extended options? | Standard warranties typically cover manufacturing defects in optics, frame integrity, and electronic components (e.g., LED and battery) for 2–3 years. Damage from accidental drops, improper handling, or unauthorized modifications is excluded. Extended warranties (up to 5 years) are available through distributors and include coverage for one-time lens recalibration or frame adjustments. Some premium brands now offer comprehensive care plans including accidental damage protection and free spare part replacements. |
| 5. How are software updates or firmware managed for smart loupes with integrated lighting controls or AR features? | Advanced loupes with adaptive lighting or augmented reality (AR) overlays utilize Bluetooth-connected companion apps for firmware updates. Clinics receive OTA (over-the-air) notifications via the app, with updates installable in under two minutes. Distributors are provided with centralized management tools for fleet updates across multiple clinic locations. Firmware logs and version tracking ensure compliance with clinical device documentation standards. |
Need a Quote for Dental Magnifying Loupes?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


