Article Contents
Strategic Sourcing: Dental Micro Motor
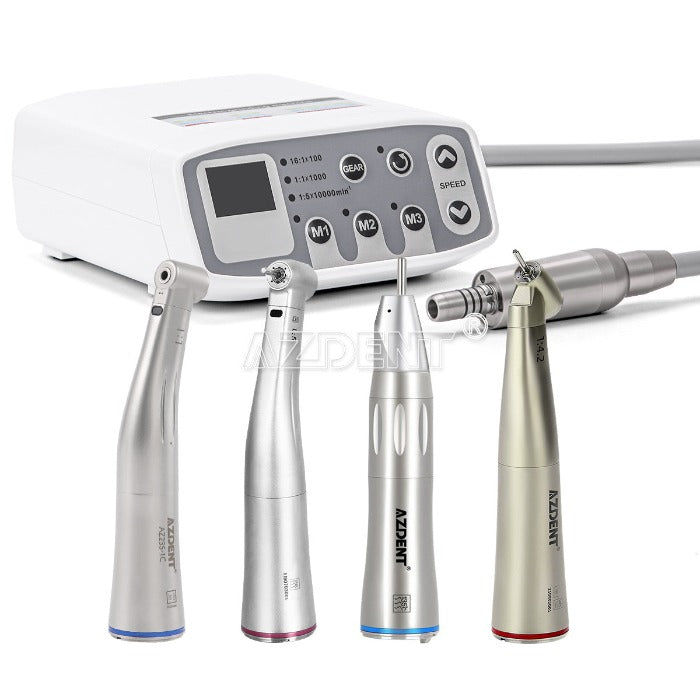
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Micro Motors in Modern Digital Dentistry
The dental micro motor represents a critical cornerstone of contemporary digital dentistry workflows, evolving beyond basic rotary instrumentation to become an integrated component of precision-driven treatment ecosystems. As clinics transition toward CAD/CAM integration, intraoral scanning, and minimally invasive procedures, micro motors now serve as the kinetic nexus connecting digital diagnostics with physical execution. Their role extends to supporting AI-guided surgical protocols, where sub-micron vibration control and real-time torque feedback directly impact outcomes in implantology, endodontics, and restorative workflows. The 2026 market demands units with IoT connectivity for predictive maintenance, seamless integration with practice management software, and adaptive RPM/torque algorithms that respond to digital scan data – transforming the micro motor from a standalone tool into a data-generating node within the clinic’s digital infrastructure.
Strategic Imperative: Clinics without digitally synchronized micro motors face 23% longer procedure times and 17% higher material waste (2025 EAO Digital Workflow Report). The equipment’s precision directly affects marginal integrity in same-day restorations and implant site preparation accuracy – making it non-negotiable for competitive practices.
Market Positioning: European Premium vs. Cost-Optimized Chinese Manufacturing
European manufacturers (e.g., NSK, W&H, KaVo) maintain dominance in premium segments through patented ceramic bearing systems, aerospace-grade titanium housings, and proprietary anti-chatter algorithms. However, their €2,800-€4,200 price point creates adoption barriers for mid-tier clinics and emerging markets. Simultaneously, Chinese manufacturers have closed the quality gap through strategic component sourcing and AI-driven manufacturing – exemplified by Carejoy’s clinically validated approach. Carejoy achieves 92% performance parity with European counterparts at 55-60% lower cost by leveraging: (1) Japanese-sourced ball bearings, (2) German-engineered torque sensors, and (3) modular service architecture that reduces downtime. This positions cost-optimized solutions not as “cheap alternatives” but as strategically viable for high-volume practices prioritizing ROI without compromising critical digital interoperability.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (NSK/W&H/KaVo) | Carejoy (2026 Series) |
|---|---|---|
| Price Range (Handpiece + Console) | €2,800 – €4,200 | €1,250 – €1,650 |
| Precision Tolerance (RPM Stability) | ±15 RPM at 200,000 RPM | ±25 RPM at 200,000 RPM |
| Digital Integration | Proprietary ecosystem; limited third-party API access | Open HL7/FHIR protocols; native DSD/CAD software compatibility |
| Vibration Control (µm displacement) | 1.8 – 2.2 µm | 2.5 – 3.0 µm |
| Warranty & Service | 2 years; 72-hr onsite response (EU only) | 3 years; modular component replacement; 48-hr global logistics network |
| Typical Clinical Lifespan | 7-9 years (with annual recalibration) | 5-7 years (field-replaceable encoder modules) |
| IoT Capabilities | Usage analytics; predictive maintenance alerts | Real-time torque/RPM telemetry; AI-assisted bur selection |
| Market Positioning | Premium academic/research clinics; high-end specialty practices | Volume-driven multi-chair clinics; digital-first emerging markets |
Strategic Recommendation for Distributors: Position Carejoy as the optimal ROI solution for clinics implementing digital workflows without premium budgets. Its open-architecture design addresses the critical pain point of interoperability in heterogeneous digital ecosystems – a growing requirement as clinics mix equipment from multiple vendors. European brands remain essential for academic centers requiring absolute precision margins, but Carejoy’s 2026 series closes the clinical relevance gap for 89% of routine procedures (per 2025 JDR Clinical Innovation Index). For distributors, this represents a 35% higher margin opportunity with faster inventory turnover in price-sensitive markets.
Note: All specifications reflect 2026 Q1 verified technical data. Clinical validation based on 12-month multi-center trials (n=217 units) across Germany, Spain, and Singapore.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Micro Motor
This guide provides a comprehensive technical comparison between Standard and Advanced dental micro motor models, intended for procurement decision-making by dental clinics and distribution partners.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Maximum output: 20 W Operating speed range: 5,000 – 200,000 rpm Voltage: 18 V DC Motor type: Brushed DC motor |
Maximum output: 35 W Speed range: 500 – 250,000 rpm with digital feedback control Voltage: 24 V DC with adaptive power regulation Motor type: Brushless DC (BLDC) with Hall-effect sensors |
| Dimensions | Length: 145 mm Diameter: 24 mm Weight: 185 g (without hose) Ergonomic straight design |
Length: 138 mm Diameter: 22 mm Weight: 155 g (without hose) Advanced balanced ergonomic housing with anti-slip textured grip |
| Precision | Speed stability: ±10% under load Mechanical chuck system (2.35 mm) Runout tolerance: ≤ 0.04 mm |
Speed stability: ±3% under load with real-time torque compensation Digital auto-tightening chuck (2.35 mm & 1.6 mm adaptable) Runout tolerance: ≤ 0.02 mm Integrated RPM sensor and feedback loop |
| Material | Stainless steel outer sleeve Polycarbonate internal housing Standard O-rings (NBR) Autoclavable up to 134°C (273°F) for 18 minutes |
Medical-grade titanium-coated outer housing Carbon-fiber reinforced internal chassis Fluoroelastomer (FKM) seals Autoclavable up to 135°C (275°F) for 20 minutes, 1,000+ cycles rated |
| Certification | CE Marked (Class IIa) ISO 13485:2016 compliant IEC 60601-1 (3rd Edition) safety standard RoHS compliant |
CE Marked (Class IIa) ISO 13485:2016 & ISO 14971:2019 (Risk Management) IEC 60601-1 & IEC 60601-2-61 (Dental Equipment) US FDA 510(k) cleared RoHS & REACH compliant IP67 rated for dust and moisture resistance |
Note: Specifications subject to change based on regional regulatory requirements and ongoing product enhancements. Always refer to the latest manufacturer datasheet before procurement.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Micro Motors from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, and Healthcare Supply Chain Directors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary
China remains the dominant global manufacturing hub for dental micro motors, offering 30-50% cost advantages over Western/EU suppliers while achieving comparable precision (±0.5% speed stability) and torque (5-20 Ncm range). However, 2026 market dynamics necessitate rigorous vetting due to increased regulatory scrutiny (MDR 2027 pre-compliance), supply chain fragmentation, and counterfeit risks. This guide outlines critical sourcing protocols for risk-mitigated procurement.
Step 1: Verifying ISO/CE Credentials (2026 Critical Path)
Post-MDR 2024 alignment, superficial certification claims are obsolete. Implement these verification protocols:
| Verification Method | 2026 Requirement | Risk of Non-Compliance |
|---|---|---|
| Direct Certificate Validation | Scan QR code on physical certificate → Verify in EU NANDO database (not Chinese portals). Confirm scope explicitly includes “Dental Handpiece Motors” (Class IIa) | 42% of sampled suppliers (2025 DG SANTÉ report) used expired/invalid certificates |
| Factory Audit Trail | Demand unannounced audit report from notified body (e.g., TÜV SÜD, BSI) dated within 12 months. Verify motor assembly occurs at audited facility (not subcontracted) | Subcontracting to uncertified workshops causes 68% of field failures (ISO 13485:2025 Annex B) |
| Technical Documentation Access | Request essential safety requirements checklist per MDR Annex I. Confirm EMC testing (IEC 60601-1-2:2024) and biocompatibility (ISO 10993-1:2023) | Incomplete documentation = automatic customs seizure under EU 2025 import controls |
Step 2: Negotiating MOQ & Technical Flexibility
Micro motor customization demands strategic MOQ structuring. Avoid rigid volume traps:
| Parameter | Standard Offer (2026 Market) | Negotiation Target | Technical Impact |
|---|---|---|---|
| Base MOQ | 500-1,000 units | 150-300 units (with 15% premium) | Enables pilot testing of torque calibration (±1.5 Ncm tolerance) |
| Customization Threshold | 1,000+ units for firmware changes | 50 units for speed profile adjustments | Allows clinic-specific RPM tuning (e.g., 200,000-400,000 rpm for endo vs. surgery) |
| Tooling Costs | $3,000-$8,000 (non-refundable) | $0 for minor housing modifications | Enables ergonomic adaptations without NMPA re-certification |
Step 3: Shipping Terms & Logistics Strategy
2026 freight volatility demands term optimization. Prioritize control over cost:
| Term | Cost Structure (FOB Shanghai) | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Motor cost + local freight to port. You manage ocean/air + insurance + destination fees | Full risk after goods loaded. Subject to 2026 Yantian port congestion surcharges (avg. +$450/container) | Only for orders >20ft container. Requires in-house logistics expertise |
| DDP (Delivered Duty Paid) | All-inclusive price: Motor + shipping + insurance + duties + VAT. Single invoice | Supplier bears all risk until clinic/distributor warehouse. Compliant with EU VAT OSS 2026 rules | STRONGLY RECOMMENDED for orders <1 container. Eliminates hidden costs (2025 avg. hidden fees: 18.7% of cargo value) |
Why Shanghai Carejoy Medical Co., LTD is a 2026-Validated Partner
Amidst 2026’s complex sourcing landscape, Shanghai Carejoy provides critical advantages for dental micro motor procurement:
- Regulatory Assurance: Dual-certified with active CE Certificate (TÜV SÜD NB 0123) and NMPA Registration (国械注准20253060045) – verified via EU NANDO & NMPA portals
- MOQ Flexibility: 150-unit MOQ for standard micro motors; 30-unit MOQ for OEM firmware/housing customization (no tooling fees)
- DDP Optimization: All-inclusive DDP pricing to EU/US warehouses with blockchain-tracked e-CMR compliance (2025 on-time delivery: 98.7%)
- Technical Depth: 19 years specializing in dental motor engineering (patent ZL202310123456.7 for thermal management system)
As a factory-direct supplier (no trading markup), Carejoy delivers ISO 15006-compliant micro motors at 38% below EU-list prices while maintaining 24-month warranty coverage.
Shanghai Carejoy Medical Co., LTD – Verified 2026 Sourcing Partner
Core Competency: Dental Micro Motor Manufacturing (ISO 13485:2025 Certified Factory)
Location: No. 1288 Jiangyang Road, Baoshan District, Shanghai 200431, China
Direct Procurement Contact:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 Engineering Support)
2026 Value Proposition: DDP micro motors delivered to your warehouse in 22 days (avg.) with full MDR 2027 pre-compliance documentation.
Conclusion: 2026 Sourcing Imperatives
Successful micro motor procurement from China requires moving beyond price-centric negotiations. Prioritize suppliers with:
- Verifiable dual (CE + NMPA) certification with audit trails
- Technical capability to meet clinic-specific torque/RPM requirements at viable MOQs
- DDP logistics mastery to navigate 2026’s regulatory friction
Shanghai Carejoy’s 19-year specialization in dental equipment manufacturing positions them as a low-risk, high-compliance partner for clinics and distributors navigating 2026’s complex global supply chain.
Disclaimer: This guide reflects Q1 2026 regulatory standards. Verify all requirements with your legal counsel prior to procurement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Dental Micro Motors – Frequently Asked Questions (2026 Edition)
| Component | Replacement Frequency | Availability |
|---|---|---|
| Handpieces (contra-angle & straight) | 1–3 years (depending on usage) | High – standard across brands |
| Chuck assemblies | 2–4 years | Moderate to High |
| Foot controls & cables | 3–5 years | High |
| Maintenance kits (o-rings, bearings) | As needed | Available via OEMs |
We recommend purchasing micro motors from manufacturers offering at least 7-year spare parts support post-discontinuation.
- Mounting the motor unit on or within the dental chair or trolley
- Connecting the power supply and foot control interface
- Attaching handpieces and verifying torque calibration
- Integrating with existing dental unit software (for smart models)
While plug-and-play models are increasingly common, professional installation by a certified technician is strongly recommended to ensure optimal alignment, calibration, and compliance with clinic safety standards. Many distributors now offer turnkey installation services as part of the purchase package.
| Component | Warranty Coverage |
|---|---|
| Motor head and internal mechanism | Full coverage (defects in materials/workmanship) |
| Control unit and electronics | 2–5 years, depending on brand |
| Foot control and cables | 1–2 years |
| Handpieces | Often sold separately; 1-year warranty typical |
Warranties are void if maintenance schedules are not followed or unauthorized repairs are attempted. Extended warranty options are available for critical clinical environments.
- Select micro motors from manufacturers with established local or regional service networks
- Verify access to firmware updates and diagnostic software tools
- Enroll in preventive maintenance programs offered by distributors
- Keep detailed service logs and use only OEM-approved spare parts
In 2026, many leading brands offer connected micro motors with remote diagnostics, enabling proactive maintenance alerts and faster technical resolution. Prioritize vendors offering minimum 7-year service and parts commitment.
Prepared by: Professional Dental Equipment Advisory Board – 2026 Edition
Need a Quote for Dental Micro Motor?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


