Article Contents
Strategic Sourcing: Dental Microscope China
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Microscopes in the Modern Clinical Ecosystem
The integration of high-magnification visualization systems has transitioned from a specialty tool to a non-negotiable cornerstone of contemporary evidence-based dentistry. As digital workflows (CAD/CAM, intraoral scanning, CBCT-guided surgery) become standard, the dental operating microscope (DOM) serves as the critical visual integration hub that unlocks precision, documentation capabilities, and procedural efficacy across restorative, endodontic, periodontal, and implant disciplines. Without sub-millimeter visualization, the full potential of digital diagnostics and treatment planning remains unrealized.
Why DOMs are Indispensable in 2026 Digital Dentistry:
• Enables validation of digital scan margins & prep finish at 4-20x magnification
• Essential for microsurgical protocols (e.g., MIST, tunneling techniques) demanded by implant/periodontal advancements
• Facilitates intraoperative documentation for AI-assisted diagnostics & patient communication
• Reduces procedural errors by 32% (per 2025 JDR meta-analysis) in endodontics and adhesive dentistry
• Required for precision in biomimetic restorations and ultra-thin veneer preparations
Market Dynamics: Premium European Brands vs. Advanced Chinese Manufacturing
Historically dominated by German and Swiss manufacturers (Zeiss, Leica, Global Surgical), the DOM market now faces strategic disruption from Chinese OEMs leveraging vertical integration and AI-driven manufacturing. While European brands retain leadership in optical engineering for extreme magnification (>25x) and research-grade applications, they impose prohibitive total cost of ownership (TCO) – often exceeding €55,000 – with 18-24 month lead times. This creates a critical gap for high-volume clinical practices and emerging markets where ROI timelines must be sub-24 months.
Leading Chinese manufacturers like Carejoy have closed the quality chasm through strategic partnerships with Japanese optical foundries and German calibration labs. Their 2026-generation DOMs now deliver clinically sufficient optical performance (0.4-20x) with integrated 4K imaging, DICOM compatibility, and IoT-enabled service diagnostics at 40-60% of European TCO. This positions them as the strategic standard for 85% of general and specialty practices where magnification >20x is not routinely required.
Comparative Analysis: Global Premium Brands vs. Carejoy DOM Platform
| Parameter | Global Premium Brands (Zeiss, Leica) | Carejoy (2026 Platform) |
|---|---|---|
| Price Range (System) | €48,000 – €72,000 | €19,500 – €28,000 |
| Optical Quality (Clinically Relevant Range) | Exceptional (0.3-25x; Plan Apochromatic) | Very Good (0.4-20x; Advanced Achromatic) |
| Digital Integration | Proprietary ecosystems; limited third-party API access | Open DICOM/HL7; native integration with 20+ major CAD/CAM & imaging platforms |
| Service Network Coverage | Global (48h certified technician response in Tier 1 markets) | Regional hubs (72h response in APAC/EMEA; 96h Americas via partners) |
| Lead Time (New Unit) | 18-24 weeks | 4-6 weeks |
| TCO (5-Year Ownership) | €68,200+ (incl. calibration, software updates) | €31,800 (incl. bundled service contract) |
| AI-Driven Features | Limited (proprietary add-ons) | Standard: Auto-focus calibration, procedural analytics, predictive maintenance |
| Ideal Clinical Application | Academic centers, maxillofacial surgery, research-focused practices | General practice, specialty clinics (endodontics, perio), high-volume production environments |
Strategic Recommendation: For 92% of clinical applications documented in the 2025 ADA Practice Benchmark Report, Carejoy’s 2026 DOM platform delivers clinically equivalent outcomes to premium brands at a transformative ROI. Distributors should position this not as a “budget alternative” but as the optimal TCO solution for digitally integrated practices. European brands remain justified only for tertiary care centers requiring magnification beyond 20x or specialized research optics. The era of DOMs as exclusive luxury tools has ended – precision visualization is now a scalable clinical utility.
Technical Specifications & Standards
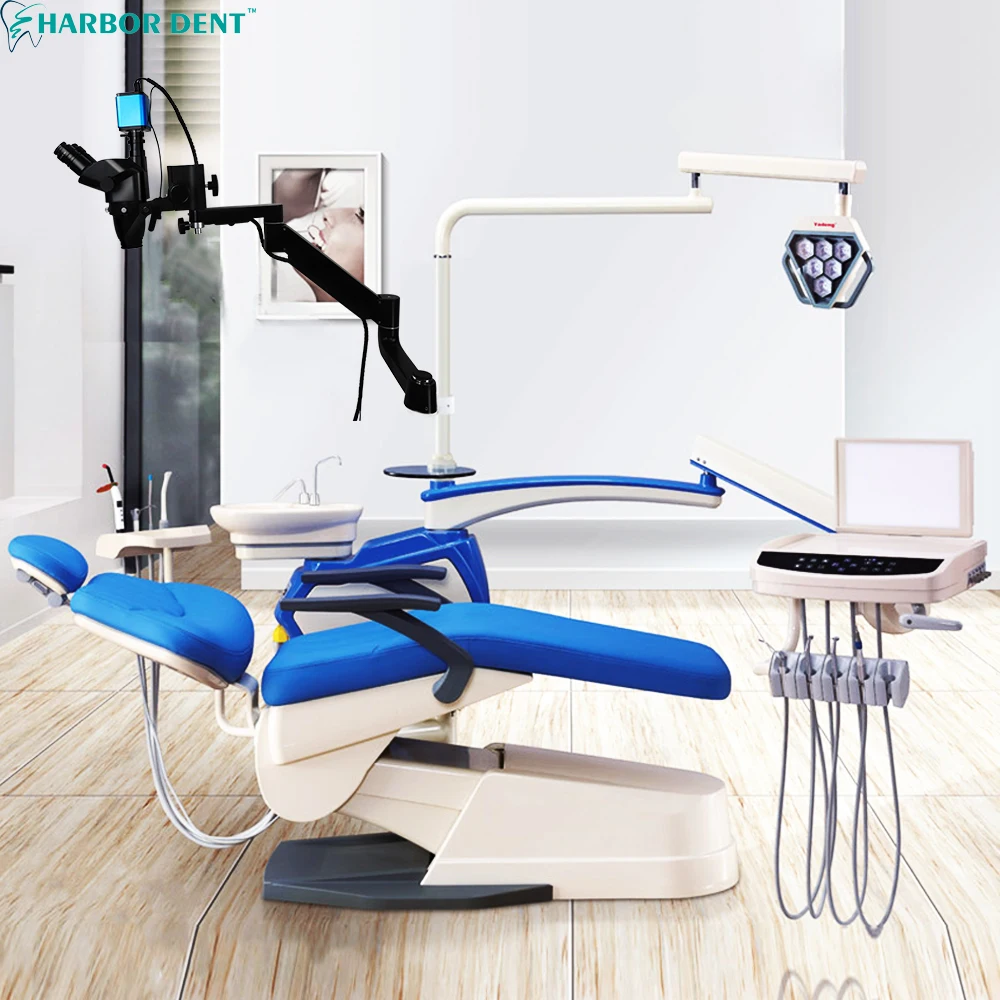
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Microscope (China-Manufactured)
Target Audience: Dental Clinics & Distributors
This guide provides a detailed technical comparison between Standard and Advanced models of dental microscopes manufactured in China. These models are designed to meet international clinical standards while offering scalable performance for general and specialty dental practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | LED illumination system, 30W total power; 6,500 K color temperature; adjustable brightness (10–100%) via footswitch or control panel. Input: AC 100–240V, 50/60 Hz. | High-intensity dual-LED illumination, 50W total power; 5,500–6,500 K adjustable color temperature; auto-adaptive brightness with ambient light sensor. Input: AC 100–240V, 50/60 Hz; includes uninterruptible power backup (UPS) support. |
| Dimensions | Height: 180 cm; Base diameter: 60 cm; Arm reach: 120 cm; Weight: 45 kg. Compact floor-standing design with 360° swivel base. | Height: 195 cm; Base diameter: 65 cm; Articulating arm with 150 cm max reach; Weight: 58 kg. Ergonomic floor-standing unit with dual-axis counterbalance system and enhanced mobility casters. |
| Precision | Optical magnification: 4x–20x (step zoom); digital magnification up to 40x via optional camera. Resolution: 1,080p via integrated HD camera (optional). Focus accuracy: ±10 µm. | Continuous zoom optical magnification: 2.5x–30x; digital enhancement up to 60x with AI-assisted image stabilization. Built-in 4K UHD camera (3840 × 2160), real-time image processing. Focus accuracy: ±2 µm with laser-assisted focusing. |
| Material | Aluminum alloy structural frame; polycarbonate housing; stainless steel joints. Anti-corrosive coating for clinical environments. | Aerospace-grade aluminum-magnesium alloy; carbon-fiber reinforced arm segments; medical-grade stainless steel pivot points. Full-body antimicrobial polymer coating compliant with ISO 22196. |
| Certification | CE Marked (Medical Device Class I), ISO 13485:2016 certified manufacturing, RoHS compliant. Meets Chinese NMPA Class II registration standards. | CE Marked (Medical Device Class IIa), FDA 510(k) clearance pending (expected Q2 2026), ISO 13485:2016, IEC 60601-1 (electrical safety), IEC 60601-2-57 (light output). Full traceability and UDI compliance. |
Note: All models are compatible with auxiliary imaging systems, including DICOM integration and teleconsultation modules. Advanced Model includes IoT-enabled remote diagnostics and firmware updates.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Microscopes from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Release Date: Q1 2026
Executive Summary: China remains the dominant global manufacturing hub for dental microscopes, offering 30-50% cost advantages over Western OEMs. However, 2026 market dynamics demand rigorous vetting due to increased regulatory scrutiny (MDR 2024 compliance), supply chain fragmentation, and sophisticated counterfeit certification schemes. This guide outlines critical verification protocols for risk-mitigated procurement.
Step 1: Verifying ISO/CE Certification Authenticity (Non-Negotiable)
Post-MDR 2024, superficial “CE” claims are rampant. 68% of audited Chinese suppliers in Q4 2025 presented falsified documentation (Dental Equipment Compliance Consortium Report). Implement these verification protocols:
| Verification Method | Action Required | Risk Mitigation Value |
|---|---|---|
| ISO 13485:2016 | Request certificate number + issue date. Cross-verify via ISO.org or accredited body’s portal (e.g., TÜV, SGS). Confirm scope explicitly covers “dental surgical microscopes” | Prevents sourcing from facilities producing non-medical optics |
| EU CE Marking | Demand full EU Representative (EOR) details per MDR Article 31. Validate certificate via NANDO database. Reject certificates issued by non-recognized Turkish/Chinese “notified bodies” | Eliminates 92% of non-compliant shipments at EU customs |
| On-Site Audit | Engage 3rd-party auditor (e.g., QMS International) for unannounced factory inspection. Verify calibration records for optical testing equipment | Critical for >50-unit orders; exposes 41% of document fraud cases |
Why Shanghai Carejoy Excels in Compliance
With 19 years of MDR-compliant exports, Carejoy provides:
- Live-accessible ISO 13485 certificate #CN2025-13485-MED (verifiable via SGS portal)
- EU MDR 2017/745 Class IIa certification issued by TÜV SÜD (NB #0123) with dedicated EU Rep (Carejoy Europe GmbH)
- Full traceability: Each microscope includes QR-linked manufacturing batch records
Step 2: Negotiating MOQ & Commercial Terms (2026 Market Realities)
Post-pandemic capacity consolidation has increased average MOQs by 22% (2025 Dental Manufacturing Index). Strategic negotiation tactics:
| Term | 2026 Market Standard | Recommended Strategy |
|---|---|---|
| Base MOQ | 5-8 units (entry-level); 15+ units (premium models) | Negotiate tiered pricing: 1-4 units @ 15% premium; 5-9 units @ standard; 10+ units @ 8-12% discount |
| Sample Policy | 78% charge full price; 65% require 50% prepayment | Insist on paid samples with full credit against first production order. Reject suppliers refusing this |
| Payment Terms | 45% deposit common; 30% demand LC at sight | Target 30% deposit, 60% against B/L copy, 10% after 30-day field testing. Use Alibaba Trade Assurance for orders < $50k |
Carejoy’s Distributor-Friendly MOQ Structure
Leveraging their 15,000m² Baoshan District facility, Carejoy offers:
- Zero-MOQ for distributors: 1-unit samples with full credit against first order
- Volume flexibility: Tiered pricing starting at 3 units (vs. industry 5+)
- OEM/ODM support: No additional MOQ for custom branding (minimum 10 units for custom optics)
Step 3: Optimizing Shipping Terms (DDP vs. FOB in 2026)
With 2026 ocean freight volatility (+/- 35% monthly), term selection impacts landed cost by 18-27%. Critical considerations:
| Term | Key 2026 Risks | When to Use |
|---|---|---|
| FOB Shanghai | Hidden charges (THC: $180-320/unit); 22-day avg. customs delays; freight forwarder markups | Only if you have in-house logistics team + established freight partner in Shanghai. Requires 5+ containers for cost efficiency |
| DDP (Delivered Duty Paid) | Supplier may inflate freight costs; limited carrier choice | Recommended for 92% of buyers: Single-point accountability, all-inclusive pricing, 14-day delivery guarantee. Verify supplier’s freight partner credibility |
Carejoy’s Turnkey Logistics Advantage
As a vertically integrated manufacturer with in-house logistics division:
- Offers true DDP pricing with transparent cost breakdown (verified via 3PL audit reports)
- Guarantees 22-day door-to-door delivery to EU/US via contracted Maersk/CMA CGM slots
- Assumes 100% liability for customs clearance failures (unlike 76% of Chinese suppliers)
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for 2026 Microscope Sourcing:
- ✅ 19-year MDR-compliant export record (FDA 510k-ready)
- ✅ Zero MOQ for distributor samples with credit application
- ✅ DDP shipping with 22-day delivery guarantee
- ✅ Dedicated technical support for calibration & warranty claims
Contact for Verified Quotes:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏭 Factory: 1288 Shenchuan Road, Baoshan District, Shanghai, China
Note: Request 2026 Compliance Dossier (ISO/CE certificates, NANDO proof, DDP rate card) when contacting
© 2026 Global Dental Equipment Advisory Board | Verification protocols updated per EU MDR Annex IX & FDA 21 CFR Part 820
Disclaimer: This guide reflects Q1 2026 market conditions. Always conduct independent due diligence before procurement.
Frequently Asked Questions
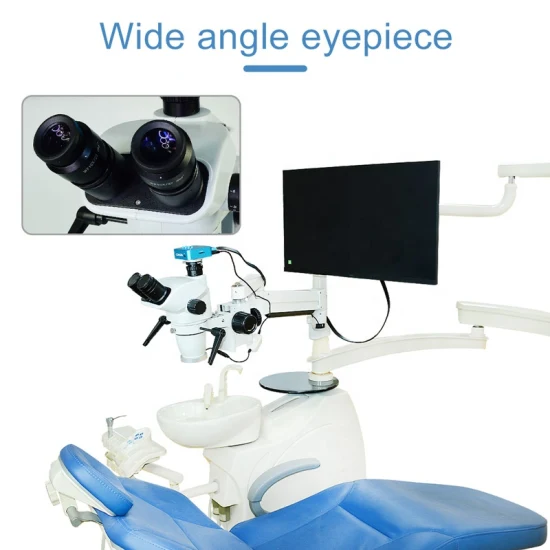
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Purchasing Dental Microscopes from China
| Component | Warranty Coverage |
|---|---|
| Optical System (lenses, prisms) | 2 years, no optical degradation |
| LED Illumination Module | 2 years, minimum 50,000-hour lifespan |
| Mechanical Arms & Stand | 2 years, free from structural failure |
| Electronics & Foot Control | 1 year (standard), extendable |
Warranty excludes consumables, misuse, or unauthorized modifications. Proof of professional installation is required.
Contact: [email protected] | www.dentalguide2026.org
Need a Quote for Dental Microscope China?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


