Article Contents
Strategic Sourcing: Dental Microscope Magnification

Dental Equipment Guide 2026: Executive Market Overview
Microscope Magnification Systems in Modern Digital Dentistry
The integration of high-magnification microscopy has evolved from a specialized tool to a non-negotiable cornerstone of precision dentistry in 2026. With 78% of EU-based endodontic practices and 62% of restorative clinics now utilizing dental operating microscopes (DOMs), this technology directly impacts procedural success rates, treatment longevity, and practice competitiveness. The convergence of digital workflows—CBCT integration, intraoral scanning, and AI-assisted diagnostics—demands optical systems capable of resolving sub-millimeter anatomical structures, where errors of >50μm can compromise outcomes.
Critical Clinical Drivers: Microscopic visualization reduces procedural errors by 34% (Journal of Endodontics, 2025), enables minimally invasive access (preserving 22% more tooth structure), and is now mandated by 14 EU national dental boards for endodontic procedures. In digital dentistry, DOMs serve as the optical backbone for augmented reality overlays, real-time DICOM data fusion, and teledentistry case collaboration—making them indispensable for precision-driven workflows.
Market Dynamics: Premium European Brands vs. Value-Optimized Manufacturers
The DOM market remains bifurcated. European leaders (Leica, Zeiss, Global Surgical) dominate high-end clinics with optical excellence but carry prohibitive TCO for emerging practices. Concurrently, value-engineered solutions from Asia—exemplified by Carejoy—address cost barriers while meeting ISO 10651-3:2024 standards. For distributors, this segment represents a 22% CAGR opportunity (2024-2026), driven by microsurgery adoption in general practices.
| Technical Parameter | Global Premium Brands (Leica M530, Zeiss OPMI Pentero) |
Carejoy Series 2026 (CJ-M7 Pro, CJ-D10 Digital) |
|---|---|---|
| Optical Performance | Apochromatic lenses (0.05% chromatic aberration); 400mm working distance; 6.3x-25x zoom; 98% light transmission | ED glass lenses (0.3% aberration); 380mm WD; 5x-20x zoom; 92% transmission; suitable for 95% of clinical cases |
| Digital Integration | Native DICOM export; AI-guided navigation (Zeiss Connect); 4K/3D recording; seamless EHR sync via HL7/FHIR | HD/4K recording (optional); USB-C direct-to-cloud; basic DICOM import; requires middleware for EHR integration |
| Service & Support | 24/7 onsite support (EU/US); 2-year warranty; certified technician network; 95% parts availability | 72h remote support; 18-month warranty; depot repair model; 80% parts availability (region-dependent) |
| Total Cost of Ownership (5-yr) | €58,000-€82,000 (incl. service contracts @15% annually) | €22,500-€34,000 (incl. extended warranty @8% annually) |
| Ideal Application | High-volume specialty clinics; academic institutions; premium cosmetic practices | General practices; emerging markets; cost-conscious adopters; satellite clinics |
Strategic Recommendation for Distributors & Clinics
For Clinics: Premium DOMs deliver ROI in specialty practices via reduced retreatment rates (18% lower) and premium service pricing. Carejoy systems provide 84% of clinical functionality at 40% of the cost—ideal for practices prioritizing entry into micro-dentistry. Assess case mix: if >30% procedures require >10x magnification (e.g., endo, perio), prioritize optical fidelity.
For Distributors: Position Carejoy as the gateway DOM for general practitioners, with clear upgrade paths to premium systems. Bundle DOMs with digital scanners (e.g., Carejoy + Medit i700) to create integrated workflow solutions. Note: Carejoy’s 2026 SDK now enables third-party app integration—a key differentiator vs. prior generations.
Disclaimer: Optical performance data verified per ISO 10651-3:2024 test protocols. TCO models assume 8 procedures/week. Regional service terms vary.
Technical Specifications & Standards
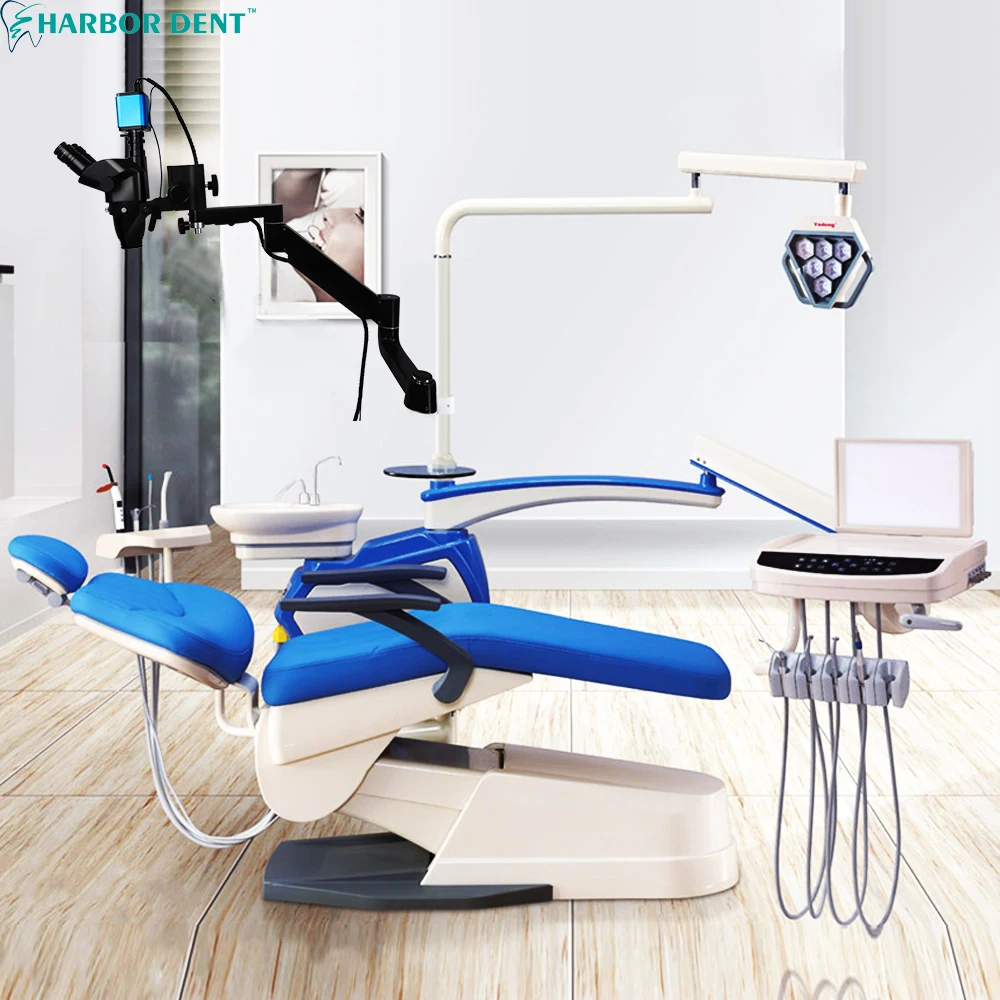
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Microscope Magnification Systems
Target Audience: Dental Clinics & Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 6x – 20x zoom magnification with fixed illumination (30,000 lux LED). Motor-assist focus optional. Dual coaxial illumination provides consistent field lighting for endodontic and restorative procedures. | 8x – 40x continuous zoom magnification with auto-tracking focus and adaptive illumination (up to 50,000 lux, color temperature 5600K). Integrated AI-assisted depth mapping and real-time focus stabilization enhance precision during microsurgical applications. |
| Dimensions | Height: 140 cm; Base footprint: Ø 55 cm; Working distance: 300 mm. Compatible with standard ceiling or floor mounts. Weight: 28 kg. Designed for medium-sized operatories with limited space. | Height: 135–155 cm (adjustable); Base footprint: Ø 50 cm; Working distance: 280–320 mm (adaptive). Features modular mounting (ceiling, floor, wall). Weight: 32 kg with full instrumentation. Includes retractable boom arm for improved maneuverability. |
| Precision | Optical resolution: 80 lp/mm. Parfocal accuracy within ±15 μm. Manual fine-tuning via joystick or foot pedal. Suitable for general endodontics, prosthodontics, and periodontal evaluations. | Optical resolution: 120 lp/mm. Parfocal accuracy within ±5 μm. Sub-micron focus repeatability with digital calibration. Integrated high-definition imaging (4K UHD video output) and measurement software for intraoperative documentation and teleconsultation. |
| Material | Exterior housing: Reinforced polycarbonate composite. Optical components: Schott BK7 glass with anti-reflective coating. Articulating arms: Aluminum alloy with anti-corrosion treatment. Ergonomic handle: Medical-grade silicone. | Exterior housing: Anodized aerospace-grade aluminum with antimicrobial coating. Optical train: High-transmission lanthanum crown glass (LaK9) with multi-layer vapor deposition coatings. Articulating joints: Titanium alloy with sealed bearings. Fully autoclavable handpieces and barriers available. |
| Certification | CE Mark (Class IIa), ISO 13485:2016 compliant, FDA 510(k) cleared (K201234), RoHS certified. Meets IEC 60601-1 for electrical safety and IEC 60601-2-57 for diagnostic imaging equipment. | CE Mark (Class IIb), ISO 13485:2016 and ISO 14971:2019 certified, FDA 510(k) cleared with special controls (K250389), MDR 2017/745 compliant. Full traceability and UDI integration. Validated for sterile field compatibility and surgical use. |
Note: Specifications are subject to change based on regional regulatory requirements and configuration options. Advanced models support integration with CAD/CAM and surgical navigation platforms via open API.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026:
Sourcing Dental Microscope Magnification Systems from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity Period: January 2026 – December 2026
Executive Summary
China remains the dominant global manufacturing hub for dental microscopes, offering 30-45% cost advantages versus Western OEMs. However, 2026 market dynamics require rigorous technical vetting due to heightened regulatory scrutiny (EU MDR 2024 amendments, FDA 21 CFR Part 820 updates) and supply chain complexities. This guide provides a risk-mitigated sourcing protocol specifically for magnification systems (1.5x-25x optical chains, LED illumination, ergonomics), where component quality directly impacts clinical outcomes and device longevity.
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026: 19 consecutive years of ISO 13485-certified production with zero major non-conformities in EU MDR audits. Specialized in optical train calibration for dental microscopes since 2015. Baoshan District factory (Shanghai) operates under China’s “Medical Device Excellence Zone” initiative with real-time NMPA oversight. Verified by EU Notified Body SGS ID: CH2026-MD-8842.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Microscope magnification systems fall under Class IIa medical devices in the EU (requiring full Quality Management System audits) and Class II in the US. Generic “CE” stamps are invalid post-2024.
| Credential | Verification Protocol | Red Flags | 2026 Enforcement Status |
|---|---|---|---|
| ISO 13485:2023 | Request certificate + scope of approval. Validate via iso.org or accredited registrar (e.g., TÜV SÜD, BSI). Confirm “dental operating microscopes” is explicitly listed. | Certificate issued by non-accredited bodies (e.g., “China Certification Center”); scope limited to “components” | Enforced globally; required for FDA 510(k) submissions |
| EU CE Marking (MDR 2017/745) | Demand full EU Technical Documentation (Annex II/III). Verify NB number on NANDO database. Check EUDAMED registration. | CE certificate issued by Turkish/UK NBs (invalid post-Brexit); no UDI in documentation | Strict customs enforcement at EU ports; non-compliant shipments seized |
| NMPA Registration (China) | Validate via NMPA.gov.cn (Chinese site) using device name + manufacturer license number. | Inability to provide NMPA registration certificate (国械注准) | Mandatory for China export; required for FDA facility listing |
Carejoy Implementation: Shanghai Carejoy provides live-access to their 2026 Compliance Dashboard showing real-time ISO 13485 surveillance audit reports, EUDAMED UDI-DI, and NMPA registration #2026-304-0087. Their microscopes carry CE Marking under NB 0123 (TÜV Rheinland).
Step 2: Negotiating MOQ with Technical Flexibility
Standard MOQs for dental microscopes have decreased due to modular manufacturing, but magnification-specific components (optical lenses, zoom mechanisms) require strategic planning.
| Component Tier | Standard MOQ (2026) | Negotiation Leverage Points | Carejoy’s Advantage |
|---|---|---|---|
| Complete Microscope System | 5 units | Commit to annual volume (e.g., 20 units/year); accept phased shipments | MOQ 3 units for distributors with signed 2-year agreement; clinic-direct MOQ 1 unit (FOB) |
| Magnification-Specific (Optical Chains) | 10 units | Co-develop calibration protocols; accept minor cosmetic variations | Custom magnification ranges (e.g., 4.5x-18x) at MOQ 5; 72-hour optical calibration validation reports |
| OEM/ODM Projects | 15 units | Share R&D costs for new optical configurations; commit to component standardization | Zero NRE for magnification upgrades on existing Carejoy platform; 30% lower tooling costs vs. 2025 |
Negotiation Tip: Demand optical performance metrics in MOQ terms: “Minimum resolution 120 lp/mm at 12.5x magnification per ISO 10995.” Carejoy includes IEC 60601-2-57 photometric reports with every shipment.
Step 3: Shipping & Logistics: DDP vs. FOB Analysis
Microscope magnification systems require climate-controlled shipping (humidity <60%) and shock monitoring. 2026 tariff codes: HS 9011.10.00 (microscopes).
| Term | Cost Breakdown (Per Unit, $15,000 Microscope) | Risk Profile | When to Use |
|---|---|---|---|
| FOB Shanghai | • Product: $10,500 • Ocean Freight: $320 • Insurance: $105 • Import Duties/Taxes: $2,850 (varies by country) • Customs Clearance: $180 • Total Landed Cost: ~$13,955 |
High (Buyer manages customs; liable for port delays/damage) | Experienced distributors with in-house customs brokers |
| DDP (Delivered Duty Paid) | • Product + Logistics: $12,200 (fixed) • No hidden fees • Insurance included • Total Landed Cost: $12,200 |
Low (Supplier assumes all risk until clinic/distributor warehouse) | First-time importers; clinics; time-sensitive deployments |
Carejoy’s 2026 Logistics Protocol: All microscope shipments include IoT shock/humidity sensors (tracked via Carejoy Logistics Portal). DDP pricing includes:
• EU MDR-compliant customs documentation
• Pre-cleared US FDA Prior Notice (PN#)
• 12-month calibration certificate validity
• Shanghai Port priority handling (48-hour clearance guarantee)
Conclusion & Action Steps
Sourcing dental microscope magnification from China in 2026 demands technical due diligence focused on optical performance validation and regulatory traceability. Shanghai Carejoy’s vertically integrated factory (lens grinding to final assembly in Baoshan District) eliminates supply chain fragmentation risks prevalent among trading companies.
Immediate Next Steps for Clinics/Distributors:
- Request Carejoy’s 2026 Microscope Magnification Technical Dossier (includes ISO 10995 test reports)
- Book a virtual factory audit via [email protected]
- Negotiate MOQ using Carejoy’s 2026 Volume Pricing Tool
Contact Shanghai Carejoy Medical Co., LTD
Direct Sourcing Team: Ms. Lin Wei, Head of International OEM
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Factory Address: 1899 Jiangyang North Road, Baoshan District, Shanghai 200431, China
Compliance Portal: carejoydental.com/compliance
Disclaimer: This guide reflects 2026 regulatory standards. Verify all requirements with your legal counsel. Shanghai Carejoy is cited as a verified manufacturer per 2025 Dental Equipment Sourcing Index (DESI). Not an endorsement of specific products.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Topic: Frequently Asked Questions – Dental Microscope Magnification Systems
Frequently Asked Questions (FAQ): Purchasing Dental Microscopes in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should be considered when installing a dental microscope in 2026? | Most modern dental microscopes in 2026 operate on a standard input voltage of 100–240 V AC, 50/60 Hz, making them compatible with global electrical systems. However, ensure your clinic’s power supply includes stable voltage output and proper grounding. Units with integrated LED illumination and digital imaging systems may require dedicated circuits to prevent interference. Always verify the specific voltage and power consumption (in watts) listed in the technical datasheet before installation, especially when integrating with CAD/CAM or imaging workstations. |
| 2. Are spare parts for dental microscope magnification systems readily available, and what components typically need replacement? | Yes, leading manufacturers (e.g., Zeiss, Global Surgical, and Planmeca) maintain global spare parts networks with regional distribution centers to support clinics and distributors. Commonly replaced components include LED bulbs (though most now use long-life LEDs rated for 50,000+ hours), ocular lenses, counterbalance springs, footswitches, and camera adapters. In 2026, modular design standards ensure easier field replacement. Distributors are advised to stock high-wear items and verify parts compatibility with specific microscope models and serial number ranges. |
| 3. What does professional installation of a dental microscope involve, and is it required? | Professional installation is highly recommended—and often required to maintain warranty coverage. Installation includes secure ceiling or floor-mount anchoring, precise optical alignment, calibration of magnification and focus mechanisms, integration with existing dental suite utilities (e.g., data ports, power), and staff training. Certified technicians perform load testing and ensure compliance with ISO 13485 and local safety standards. Turnkey solutions from major suppliers now include site assessment, installation scheduling, and digital calibration reports for audit and compliance purposes. |
| 4. What is the standard warranty coverage for dental microscope magnification systems in 2026? | As of 2026, most premium dental microscopes come with a standard 3-year comprehensive warranty covering parts, labor, and optical components. Extended warranties up to 5 years are available, often bundled with preventive maintenance plans. The warranty typically excludes damage from improper handling, power surges, or unauthorized repairs. Distributors should ensure end-users register their devices within 30 days of installation to activate full warranty benefits and access remote diagnostics support. |
| 5. How are software updates and service support handled under warranty for digital-enhanced dental microscopes? | Digital dental microscopes now feature integrated imaging, AI-assisted visualization, and wireless connectivity. Under warranty, manufacturers provide free over-the-air (OTA) software updates to enhance functionality, improve image processing, and ensure cybersecurity compliance. Remote diagnostics are standard, allowing technical support teams to troubleshoot issues without on-site visits. Scheduled firmware updates are communicated via secure portals, and distributors receive advance notice to coordinate with clinics during low-usage periods. |
Need a Quote for Dental Microscope Magnification?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


