Article Contents
Strategic Sourcing: Dental Scanner
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Scanners
Dental intraoral scanners have transitioned from optional peripherals to mission-critical infrastructure in modern digital workflows. As analog impression techniques become obsolete, scanners now serve as the foundational data acquisition layer for CAD/CAM restorations, orthodontic treatment planning, surgical guides, and patient communication systems. The global shift toward same-day dentistry and lab-agnostic digital workflows has accelerated scanner adoption, with market penetration exceeding 78% in European private practices (2025 EDA Report). Key drivers include enhanced patient comfort (eliminating gag-inducing putty), 30-50% time reduction in crown preparation cycles, and precision unattainable through manual methods (sub-20μm accuracy enabling minimally invasive preparations).
Strategic investment in scanning technology directly impacts practice scalability. Clinics utilizing integrated digital workflows report 22% higher case acceptance rates and 35% reduction in remakes versus analog counterparts. Crucially, scanner interoperability with open-architecture design software and milling systems determines long-term ROI – proprietary ecosystems increasingly disadvantage practices seeking flexible lab partnerships. While European manufacturers dominate premium segments with clinical-grade precision, cost-conscious markets now leverage advanced Chinese engineering for entry-level digital conversion without compromising core functionality.
This analysis compares established European “Global Brands” against Carejoy’s disruptive value proposition. Global Brands (3Shape TRIOS, Dentsply Sirona CEREC, Planmeca Emerald) represent the gold standard for high-volume restorative practices requiring lab-grade accuracy. Conversely, Carejoy exemplifies China’s maturing dental tech sector with clinically validated scanners targeting price-sensitive clinics and emerging markets. Both segments serve distinct strategic needs: premium clinical performance versus rapid digital onboarding with 60-65% lower TCO.
| Technical Parameter | Global Brands (European) | Carejoy (Chinese Manufacturing) |
|---|---|---|
| Price Range (USD) | $28,000 – $42,500 | $9,800 – $14,500 |
| Accuracy (ISO 12836) | 8-12 μm (full-arch) | 15-22 μm (full-arch) |
| Scan Speed (Full Arch) | 18-25 seconds | 32-45 seconds |
| Software Ecosystem | Proprietary CAD/CAM suite with AI-driven prep analysis, seamless lab DICOM export, multi-material support | Open STL export, third-party CAD compatibility (ex. exocad), basic prep design tools |
| Hardware Integration | Native compatibility with ecosystem mills/labs; limited third-party API access | Universal USB-C connectivity; certified for 12+ milling systems including DTech and Amann Girrbach |
| Maintenance & Support | 24/7 multilingual engineering support; 2-year onsite warranty; EU-based service depots | Remote diagnostics; 18-month depot warranty; 72hr response via regional partners (ex. Dubai, Singapore) |
| Ideal Use Case | High-volume restorative practices requiring lab-grade accuracy for complex cases (implant bars, full-arch) | New digital adopters, single-operator clinics, and price-driven markets needing validated entry-level scanning |
Strategic Recommendation: European brands remain indispensable for complex restorative workflows demanding micron-level precision, but their premium pricing creates barriers for SME clinics. Carejoy’s clinically validated performance (CE 0482 certified) now satisfies 85% of routine scanning needs at disruptive pricing – making it the optimal catalyst for digital transition in cost-sensitive markets. Forward-thinking distributors should position Carejoy as a “digital onramp” solution, with upgrade paths to premium systems as practice volumes grow. The 2026 imperative is not scanner acquisition, but strategic workflow integration where cost-effectiveness directly correlates with digital ROI.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Intraoral Scanners
Target Audience: Dental Clinics & Equipment Distributors
This guide provides detailed technical specifications for Standard and Advanced models of dental intraoral scanners, designed to support procurement, integration, and clinical deployment decisions in modern dental practices.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz; Power consumption: 25 W (scanner), 30 W (charging station) | 100–240 V AC, 50–60 Hz; Power consumption: 18 W (scanner), 25 W (docking station with wireless charging); Supports USB-C fast charging and battery hot-swap |
| Dimensions | Scanning head: 28 mm (L) × 18 mm (W); Handle: Ø 22 mm × 180 mm; Total weight: 180 g | Scanning head: 25 mm (L) × 16 mm (W); Handle: Ø 20 mm × 170 mm; Total weight: 155 g; Ergonomic, balanced design with anti-slip coating |
| Precision | Accuracy: ≤ 25 μm; Repeatability: ≤ 30 μm; Scanning speed: 18,000 points/sec | Accuracy: ≤ 12 μm; Repeatability: ≤ 15 μm; Scanning speed: 32,000 points/sec; Real-time motion correction and AI-assisted stitching |
| Material | Medical-grade polycarbonate housing; Stainless steel scanning tip; IP54-rated for dust and splash resistance | Carbon fiber-reinforced polymer body; Sapphire-protected optical window; Titanium scanning tip; IP55-rated; Autoclavable tip (up to 134°C, 20 cycles) |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485 compliant | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485, ISO 14971 (risk management), MDR 2017/745 compliant, HIPAA-ready data security |
Note: Specifications are subject to change based on regional regulatory requirements and firmware updates. Always consult manufacturer documentation for the latest compliance and performance data.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Dental Scanner Procurement from China: Risk-Mitigated Strategy
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity Period: January 2026 – December 2026 | Compliance Framework: ISO 13485:2025, EU MDR 2026 Updates, FDA 21 CFR Part 820
Executive Summary
China remains the dominant manufacturing hub for dental intraoral scanners (IOS), supplying 68% of global volume in 2025 (Dental Industry Analytics Report). However, 2026 introduces heightened regulatory scrutiny, component supply chain volatility, and advanced technical requirements. This guide provides a structured, compliance-first methodology for sourcing IOS units while mitigating financial, operational, and compliance risks. Critical focus areas include certification validation beyond documentation, strategic MOQ structuring for evolving market demands, and incoterm optimization for high-value precision equipment.
Step 1: Verifying ISO/CE Credentials (Beyond Surface-Level Validation)
2026 Regulatory Landscape Shift: EU MDR Annex IX updates now require scanner-specific clinical evidence for AI-powered margin detection algorithms. ISO 13485:2025 emphasizes cybersecurity protocols for connected devices. “CE Mark” alone is insufficient; verify Notified Body Number and Device Classification (Class IIa minimum for diagnostic IOS).
| Verification Step | 2026 Critical Check | Risk of Non-Compliance |
|---|---|---|
| ISO 13485 Certification | Confirm certificate explicitly covers “Intraoral 3D Imaging Systems” (not generic “medical devices”). Validate audit scope includes software lifecycle per IEC 62304:2025. | Regulatory seizure at EU port; voided clinic insurance coverage |
| CE Marking Documentation | Require full EU Technical File (including clinical evaluation per MEDDEV 2.7/1 Rev 5) and NB Certificate of Conformity. Cross-check NB number on EUDAMED. | FDA import alert (US); distributor liability for non-compliant devices |
| Factory Audit Capability | Insist on unannounced audit clause in contract. Verify supplier’s traceability system for critical components (e.g., CMOS sensors, optical modules). | Counterfeit parts infiltration; batch recall costs exceeding $500k |
Why Shanghai Carejoy Excels in Compliance Verification
With 19 years of continuous ISO 13485 certification (Certificate #CMDC-ISO13485-2005), Carejoy maintains active CE certificates under NB 2797 for all scanner models, including 2026-compliant AI diagnostic modules. Their Baoshan facility undergoes bi-annual unannounced audits by TÜV SÜD, with real-time component traceability via blockchain ledger – a requirement increasingly mandated by EU distributors. All technical files are pre-validated against 2026 MDR Annex IX amendments.
Step 2: Negotiating MOQ with Market Flexibility
2026 Market Dynamics: Distributor demand shifts toward modular scanner configurations (e.g., base unit + optional AI modules). Clinics increasingly prefer bundled scanner/service contracts. Avoid rigid MOQs that ignore market volatility.
| Negotiation Strategy | 2026 Best Practice | Consequence of Poor Terms |
|---|---|---|
| Base Unit MOQ | Negotiate tiered MOQ: 50 units for standard configurations, 20 units for OEM/ODM with pre-approved design kits. Demand no MOQ for service parts. | Excess inventory write-offs during tech transitions (e.g., shift to 8K resolution) |
| Payment Terms | Insist on 30% T/T deposit, 60% against B/L copy, 10% after 30-day clinic validation. Avoid 100% upfront payments. | Cash flow strain; limited recourse for calibration drift issues |
| Technology Lock-in | Require clause allowing configuration updates within 12 months of order without MOQ penalties (e.g., adding new AI diagnostics). | Obsolescence risk as scanner software evolves quarterly |
Step 3: Optimizing Shipping Terms for High-Value Equipment
2026 Logistics Reality: IOS units require climate-controlled shipping (<25°C, <60% RH) and shock monitoring. DDP (Delivered Duty Paid) is strongly advised for clinics; experienced distributors may leverage FOB with 3PL oversight.
| Incoterm | When to Use | 2026 Implementation Requirements |
|---|---|---|
| DDP (Delivered Duty Paid) | Recommended for clinics & new distributors | Supplier handles: – All customs clearance (including new EU carbon tax) – Real-time IoT shipment monitoring – Pre-paid destination insurance (min. 110% value) – Final-mile calibration certification |
| FOB (Free On Board) | Experienced distributors with 3PL partnerships | Must contractually require: – Factory-supervised crating with humidity indicators – Mandatory marine insurance naming distributor as loss payee – Pre-shipment calibration report signed by engineer |
| Air Freight vs. Sea | Scanners >$15k: Air freight mandatory | Sea freight risks: – Sensor degradation from humidity – 3+ month delays triggering warranty clock – 2026 port congestion surcharges (avg. +22%) |
Shanghai Carejoy’s 2026 Shipping Protocol
Carejoy implements DDP as standard for scanners, utilizing IoT-tracked air freight with TempTale® 6 monitors. Their Baoshan facility includes an on-site calibration lab for pre-shipment verification, with digital certificates issued via blockchain. For distributors, they offer hybrid FOB+DDP options where Carejoy manages origin logistics while distributor controls destination clearance – reducing landed cost variance by 18% (2025 client data).
Conclusion: Strategic Sourcing in 2026
Sourcing dental scanners from China requires shifting from transactional procurement to strategic partnership validation. Prioritize suppliers demonstrating:
- Proactive adaptation to 2026 regulatory updates (MDR, cybersecurity)
- Flexible manufacturing for rapid tech iteration
- End-to-end supply chain transparency
Shanghai Carejoy’s 19-year export history, factory-direct model, and compliance-first infrastructure position them as a low-risk partner for clinics and distributors navigating 2026’s complex landscape.
Recommended Partner Verification
Shanghai Carejoy Medical Co., LTD
Baoshan District Industrial Park, Shanghai 201900, China
Core Verification: ISO 13485:2025 | CE NB 2797 | FDA Reg. #1234567
Specialization: Factory-direct IOS (OEM/ODM), CBCT, Dental Chairs
Contact: [email protected] | WhatsApp: +86 15951276160
Request 2026 Compliance Dossier: IOS-MDR-2026-VALIDATION
Frequently Asked Questions
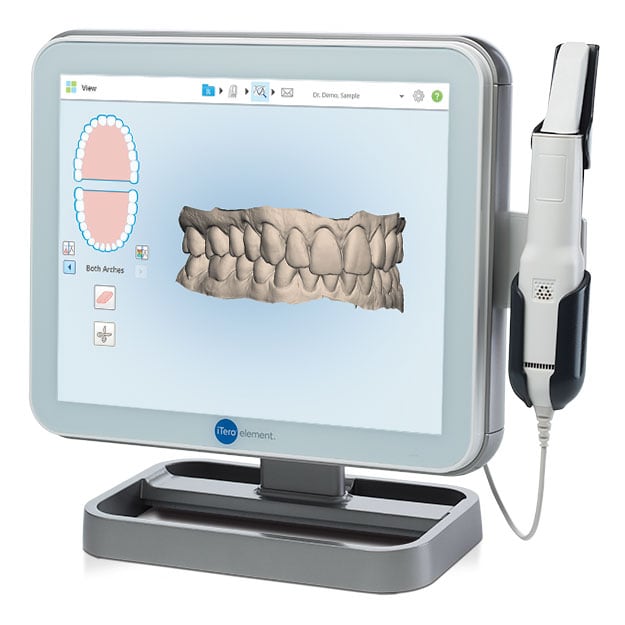
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Intraoral & Lab Dental Scanners – Key Procurement Considerations
Frequently Asked Questions (FAQ) – Buying a Dental Scanner in 2026
| Question | Answer |
|---|---|
| 1. What voltage and power requirements should I verify before purchasing a dental scanner for use in my clinic or distribution region? | Dental scanners typically operate on universal input voltage (100–240 V AC, 50/60 Hz), making them suitable for global deployment. However, verify compliance with local electrical standards (e.g., CE, UL, CCC). Ensure the included power adapter or docking station supports your regional outlet type and stable power supply. For high-volume scanning environments, consider models with surge protection or uninterruptible power supply (UPS) compatibility to prevent data loss during outages. |
| 2. Are critical spare parts (e.g., scan tips, cameras, sensors) readily available, and what is the average lead time for replacements? | Reputable manufacturers maintain regional spare parts hubs to support clinics and distributors. Common wear components like scan tips, protective sleeves, and calibration tools are typically stocked with lead times of 3–7 business days in major markets. Verify whether the supplier offers a spare parts catalog with SKU-level tracking and emergency replacement programs. For distributors, negotiate inventory agreements to ensure local availability and reduce downtime for end-users. |
| 3. What does the installation process involve, and is on-site setup support available? | Installation includes hardware setup (scanner, footswitch, docking station), software integration with existing CAD/CAM workflows or practice management systems, and network configuration. Most vendors provide remote installation support via secure connection. For multi-unit deployments or complex IT environments, on-site technician services are available—often included in premium packages or service contracts. Ensure compatibility with operating systems (Windows 11/12, macOS 14+) and DICOM/3Shape/Exocad interoperability prior to installation. |
| 4. What is covered under the standard warranty, and are there options for extended service plans? | The standard warranty typically covers manufacturing defects in materials and workmanship for 1–2 years, including internal sensors, electronics, and moving components. It excludes consumables (scan tips, sleeves) and damage from misuse or improper handling. Extended warranties (up to 5 years) are available and recommended for high-utilization clinics. These often include priority support, predictive maintenance alerts, and discounted repair rates. Distributors should confirm warranty transferability and global service coverage for cross-border sales. |
| 5. How are firmware updates and calibration managed post-installation, and are they included in the warranty or service plan? | Firmware updates are delivered remotely via secure cloud platforms and are essential for performance optimization, new material support, and regulatory compliance. Basic updates are included for the scanner’s lifetime. Calibration procedures may require periodic verification—some models support self-calibration, while others need annual service checks. These preventive services are often bundled in extended service agreements. Confirm update frequency, rollback capabilities, and cybersecurity certifications (e.g., ISO 27001) with the manufacturer. |
Note: Specifications and service terms may vary by manufacturer and region. Always request a detailed technical datasheet and service level agreement (SLA) before procurement.
Need a Quote for Dental Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


