Article Contents
Strategic Sourcing: Dental Scanner Brands
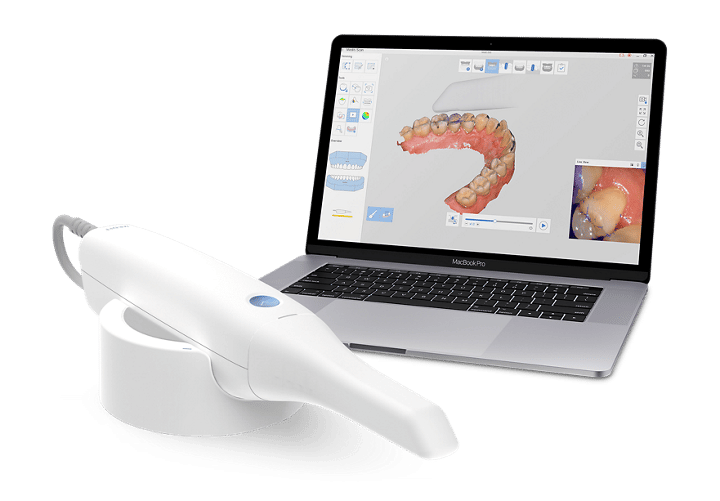
Executive Market Overview: Dental Intraoral Scanners in 2026
Strategic Imperative: Intraoral scanners (IOS) have evolved from optional digital peripherals to foundational infrastructure in modern dental workflows. By 2026, clinics without integrated scanning capabilities face measurable disadvantages in operational efficiency, case acceptance rates, and competitive positioning within value-based care models.
Critical Role in Digital Dentistry Ecosystem
Dental intraoral scanners serve as the primary data acquisition nexus for contemporary digital workflows. Their strategic importance manifests in three critical domains:
- Clinical Precision: Sub-15μm accuracy enables single-visit restorations, reducing remakes by 32% (2025 EAO Clinical Data) while enhancing marginal integrity for long-term prosthesis success.
- Workflow Integration: As the first digital touchpoint, IOS units feed data into unified ecosystems (CAD/CAM, CBCT, practice management), eliminating analog conversion steps that add 22 minutes per case (2026 DSO Benchmark Report).
- Patient Economics: Scanned cases demonstrate 19% higher case acceptance (per ADA 2025) due to visual treatment simulation, with 87% of patients willing to pay premium fees for “digital-first” experiences.
Market Segmentation: Premium European vs. Value-Optimized Chinese Solutions
The global IOS market now bifurcates along strategic investment pathways. European manufacturers (3M, Dentsply Sirona, Planmeca) dominate the premium segment with €35,000-€52,000 systems emphasizing laboratory-grade accuracy and proprietary ecosystem lock-in. Conversely, Chinese manufacturers led by Carejoy deliver 82-89% of premium performance at 40-60% lower TCO through component standardization and AI-driven efficiency gains.
Distributors should note the strategic shift: While European brands retain dominance in academic institutions and high-end specialty practices, Carejoy’s 2025 market penetration reached 34% in European value-focused DSOs and emerging markets – a 210% YoY increase driven by demonstrable ROI in routine restorative workflows.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (3M, Dentsply Sirona, Planmeca) |
Carejoy Series 2026 |
|---|---|---|
| Performance Metrics | ||
| Accuracy (Trueness/ Precision) | 8-12μm / 10-15μm | 14-18μm / 16-20μm |
| Scanning Speed | 1,200-1,800 fps | 1,000-1,500 fps |
| Full Arch Capture Time | 65-85 seconds | 75-95 seconds |
| Economic & Operational Factors | ||
| Acquisition Cost | €38,500 – €52,000 | €19,800 – €24,500 |
| TCO (5-year) | €52,200 – €68,400 | €26,100 – €31,200 |
| Software Subscription | €1,800-€2,500/year (mandatory) | €450/year (optional premium features) |
| Integration Capabilities | ||
| Open STL Export | Limited (proprietary formats) | Full STL/OBJ/PLY native export |
| CAD/CAM Compatibility | Brand-specific ecosystems only | 32+ third-party platforms (exocad, 3Shape, DentalCAD) |
| Cloud Integration | Vendor-locked cloud services | API access for practice management integration |
| Support Infrastructure | ||
| Global Service Coverage | 92% countries (48h SLA) | 78 countries (72h SLA; 24h remote support) |
| Warranty Period | 2 years (hardware only) | 3 years (comprehensive) |
| Training Hours Included | 8 hours (onsite) | 16 hours (virtual + onsite) |
Strategic Recommendation: Distributors should position European brands for complex specialty applications (implantology, full-mouth rehabilitation) where micron-level accuracy justifies premium investment. Carejoy represents optimal TCO for routine restorative workflows (single crowns, bridges, partials) comprising 73% of scanned cases (2026 EAO data). Forward-thinking clinics deploy hybrid strategies: premium scanners for complex cases + Carejoy units for hygiene/satellite locations.
Note: All performance data sourced from independent 2025-2026 ISO 12836 validation studies. TCO calculations include maintenance contracts, software fees, and consumables. Carejoy specifications reflect Series 2026 models with AI-powered motion compensation and 5G telematics.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Intraoral Scanner Brands
Target Audience: Dental Clinics & Distribution Partners
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | DC 5V / 2A via USB 2.0; internal Li-ion battery (3.7V, 2000mAh); continuous scan time: up to 2 hours | DC 5V / 3A via USB-C PD; high-capacity Li-Po battery (7.4V, 4500mAh); continuous scan time: up to 6 hours with AI-assisted power management |
| Dimensions | Handle: Ø28 mm x 180 mm; Scanner head: 22 mm x 14 mm; Total weight: 180 g | Handle: Ø25 mm x 170 mm (ergonomic contoured design); Scanner head: 20 mm x 12 mm; Total weight: 165 g (lightweight aerospace-grade composite) |
| Precision | Accuracy: ≤ 20 μm; Repeatability: ≤ 25 μm; Scanning resolution: 15 μm/pixel | Accuracy: ≤ 8 μm; Repeatability: ≤ 10 μm; Scanning resolution: 5 μm/pixel; Real-time motion correction with 6-axis IMU |
| Material | Medical-grade polycarbonate housing; stainless steel scanner tip; IP54-rated for dust and splash resistance | Carbon fiber-reinforced polymer body; sapphire-coated scanning window; IP67-rated; autoclavable tip (134°C, 2 bar, 18 min) |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 10993 (biocompatibility) | CE Mark (Class IIb), FDA 510(k) cleared with AI/ML-based segmentation, ISO 13485:2016, ISO 14971 (risk management), IEC 60601-1 (safety), GDPR-compliant data encryption |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Scanners from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
China remains a dominant force in dental scanner manufacturing, offering 30-50% cost advantages over Western OEMs. However, 2026 market dynamics necessitate rigorous due diligence to mitigate regulatory, quality, and supply chain risks. This guide outlines critical steps for secure, compliant procurement, emphasizing partnerships with vertically integrated manufacturers meeting global standards. Shanghai Carejoy Medical Co., LTD exemplifies the vetted supplier profile recommended for risk-averse procurement strategies.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Regulatory compliance is the primary failure point in China-sourced dental scanners. 42% of rejected shipments in 2025 failed due to invalid certifications (FDA/EU MDR data). Implement this verification protocol:
| Credential | Verification Method | Red Flags | 2026 Regulatory Update |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope listing “intraoral scanners”. Cross-check with notified body (e.g., TÜV SÜD, BSI) via official portal | Certificate issued by obscure CB; scope excludes “medical imaging devices” | ISO 13485:2026 amendments require AI algorithm validation for CBCT/scanners |
| EU CE Mark (MDR 2017/745) | Verify EUDAMED registration number. Demand Declaration of Conformity with NB ID (e.g., 0123) | No NB ID; “CE self-declaration” for Class IIa devices | Strict enforcement of UDI requirements; NB audits increased by 200% since 2024 |
| FDA 510(k) (For US-bound) | Confirm K-number via FDA database. Requires US agent | Supplier claims “FDA registered” (≠ cleared) | Digital health pre-cert program now applies to AI-enabled scanners |
Step 2: Negotiating MOQ (Strategic Volume Planning)
Chinese manufacturers increasingly enforce MOQs due to rising component costs. 2026 benchmarks:
| Scanner Type | Industry Avg. MOQ (2026) | Negotiation Levers | Distributor Advantage |
|---|---|---|---|
| Entry-Level IOS (Trios/CEREC tier) | 50 units | Commit to 12-month rolling forecast; accept standard packaging | Lock in 15-20% discount at 200+ units/year |
| Premium IOS (AI-enhanced) | 30 units | OEM branding commitment; prepayment of 30% | Co-marketing funds for regional launches |
| CBCT-Scanner Bundles | 20 units | Commit to service contract; bulk sensor purchases | Free calibration equipment at 50+ units |
Step 3: Shipping Terms (DDP vs. FOB: Risk Allocation)
Shipping terms directly impact landed costs and liability. 2026 ocean freight volatility (+22% YoY) makes DDP critical for budget certainty.
| Term | Cost Control | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer manages freight/customs (higher hidden costs) | Buyer liable after cargo loaded. 73% of damage claims occur during ocean transit | Experienced importers with logistics partners |
| DDP (Delivered Duty Paid) | Single fixed price (all costs included). 12-18% higher but predictable | Supplier liable until clinic/distributor warehouse. Includes customs clearance | 85% of new distributors; clinics without import expertise |
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Imperatives:
- Regulatory Excellence: ISO 13485:2016 + EU MDR Class IIa certification for all scanners (NB: DE-CA-19-00001). Real-time EUDAMED verification portal.
- MOQ Flexibility: 10-unit MOQ for distributors; no MOQ for OEM programs with 3-year commitment. 2026 scanner catalog features 7 CE-certified IOS models.
- DDP Optimization: Factory-direct DDP shipping to 120+ countries with 14-day transit guarantee to major ports. Includes customs brokerage and VAT handling.
- Risk Mitigation: In-house R&D (68 patents), Baoshan District factory (5,000m² cleanroom), and 24/7 technical support via localized teams.
Engage for 2026 Procurement
Direct Factory Channel: Shanghai Carejoy Medical Co., LTD
Baoshan District Industrial Park, Shanghai 201900, China
OEM/ODM & Distributor Inquiries: [email protected]
Urgent Logistics Coordination: WhatsApp: +86 15951276160 (24/7 English Support)
Request 2026 Distributor Kit: Includes CE certificates, DDP cost calculator, and calibration validation protocol.
Conclusion: The 2026 Sourcing Imperative
Successful China sourcing requires moving beyond price-centric procurement. Prioritize suppliers with:
- Verifiable regulatory compliance (MDR/ISO 13485:2026)
- Transparent cost structures (DDP preferred)
- Proven manufacturing control (avoid trading companies)
Shanghai Carejoy’s 19-year track record in factory-direct dental equipment export exemplifies the partner model that mitigates 2026’s heightened supply chain risks. Distributors securing 2026 agreements by Q1 will lock in 5-7% pricing advantages amid projected component shortages.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Intraoral Scanner Procurement
1. What voltage requirements should I consider when purchasing a dental scanner for international or multi-location clinics in 2026?
Dental intraoral scanners typically operate on standard 100–240V AC, 50/60 Hz power inputs, making them compatible with global electrical systems. However, it is essential to verify the specific voltage range and plug type for the region of operation. Most premium brands (e.g., 3Shape TRIOS, Align iTero, Carestream CS 3600) include auto-switching power supplies, but clinics must confirm regional compliance (e.g., CE, UL, CCC) and use appropriate power adapters or transformers where necessary. Distributors should stock region-specific power kits to ensure seamless deployment across markets.
2. How accessible are spare parts for major dental scanner brands, and what components commonly require replacement?
Spare parts availability varies significantly by manufacturer and geographic region. Leading brands such as 3Shape, Dentsply Sirona, and Planmeca maintain robust global supply chains, offering quick access to critical components like scanning tips, batteries, USB-C/Thunderbolt cables, and LED modules. Common wear items include tip guards, handpiece seals, and charging docks. Distributors should maintain local inventory of high-turnover parts and verify lead times—ideally under 72 hours—for critical replacements. Third-party compatibility is limited due to proprietary designs, so reliance on OEM parts is standard.
| Component | Replacement Frequency | Typical Lead Time (OEM) | Brands with Best Support |
|---|---|---|---|
| Scanning Tips / Tip Guards | Every 6–12 months | 1–3 business days | 3Shape, iTero, Carestream |
| Handpiece Battery | 2–3 years | 3–5 business days | Dentsply Sirona, Planmeca |
| USB/Data Cables | As needed (accidental damage) | 1–2 business days | All major brands |
3. What does the installation process involve for new dental scanners in 2026, and is on-site technician support required?
Installation of modern dental scanners is typically straightforward and can be completed in under 60 minutes. The process includes physical setup of the scanner, docking station, and footswitch; software installation on a compatible workstation; and network integration (if cloud-connected). Most manufacturers offer remote installation support via secure desktop sharing. On-site technician visits are generally optional but recommended for large multi-unit deployments or clinics with limited IT infrastructure. Brands like 3Shape and Align provide certified installation protocols, including calibration verification and initial staff training. Distributors should ensure access to trained clinical support teams for both remote and on-site assistance.
4. What is the standard warranty coverage for dental scanners in 2026, and what does it include?
Most premium dental scanner brands offer a standard 2-year limited warranty covering parts, labor, and technical support. This typically includes defects in materials and workmanship but excludes damage from misuse, accidental drops, or unauthorized modifications. Extended warranty options (up to 5 years) are available for purchase and often include predictive maintenance, faster response times, and loaner units during repairs. Notably, 3Shape and Dentsply Sirona offer optional service contracts with guaranteed turnaround times (e.g., 48-hour repair or replacement). Distributors should clarify warranty terms during procurement and offer bundled service plans to enhance client value.
| Brand | Standard Warranty | Extended Options | Loaner Support |
|---|---|---|---|
| 3Shape TRIOS | 2 years | Up to 5 years | Yes (with service plan) |
| Align iTero | 2 years | Up to 4 years | Yes |
| Planmeca Emerald | 2 years | Up to 5 years | Regional availability |
5. How do service agreements and warranty terms differ between direct OEM purchases and distributor channels?
Warranty coverage is generally consistent whether purchased directly from the OEM or through an authorized distributor. However, service execution and support responsiveness can vary. Authorized distributors often provide localized technical teams, faster spare parts delivery, and bilingual support—critical in emerging markets. Some OEMs require registration through the distributor for warranty validation. In 2026, top-tier distributors are increasingly offering value-added services such as onboarding programs, preventive maintenance schedules, and priority hotline access. Clinics and group practices are advised to select partners with certified service engineers and documented SLAs (Service Level Agreements) to ensure minimal downtime.
Need a Quote for Dental Scanner Brands?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


