Article Contents
Strategic Sourcing: Dental Scanning Machine
Dental Equipment Guide 2026: Executive Market Overview
Intraoral Scanning Systems – The Digital Cornerstone of Modern Dentistry
The global intraoral scanner (IOS) market has evolved from a premium differentiator to a non-negotiable component of competitive dental practice infrastructure. Valued at USD $2.8B in 2025 (CAGR 12.3%), this segment is projected to reach $4.1B by 2028. Driven by the irreversible shift toward digital workflows, IOS adoption is no longer optional for clinics seeking operational efficiency, clinical precision, and patient retention in the premium and mid-market segments.
Criticality in Modern Digital Dentistry: IOS systems eliminate traditional impression materials, reducing material costs by 18-22% annually while accelerating treatment cycles by 30-40%. They serve as the foundational data capture node for integrated digital workflows – directly interfacing with CAD/CAM units, treatment planning software, and teledentistry platforms. Clinics reporting full IOS integration demonstrate 23% higher case acceptance rates for complex restorations and 35% reduction in remakes versus analog workflows (2025 EDA Practice Economics Report). Patient demand for “digital smiles” (same-day restorations, virtual previews) now influences 68% of new patient acquisition decisions.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The IOS market bifurcates into two strategic segments:
- Premium European Brands (Dentsply Sirona, 3Shape, Planmeca): Representing 62% of installed base in Western Europe and North America. These systems command 35-50% price premiums based on legacy reputation, sub-15μm accuracy validation, and deep ecosystem integration. Target: High-volume specialty clinics and corporate DSOs prioritizing seamless workflow integration over upfront cost.
- Value-Optimized Manufacturers (Carejoy, et al.): Gaining 28% YoY traction in emerging markets and value-conscious private practices. Led by Chinese innovators like Carejoy, these systems deliver 85-90% of premium functionality at 40-60% lower TCO. Target: Independent clinics, new graduates, and distributors servicing price-sensitive markets without compromising essential clinical capabilities.
Technology & Value Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Dentsply Sirona, 3Shape, Planmeca) |
Carejoy Technology |
|---|---|---|
| Typical Acquisition Cost (2026) | €35,000 – €55,000+ (scanner only) | €18,500 – €24,900 (all-inclusive) |
| Trueness/Accuracy (ISO 12836) | Sub-15μm (validated in independent studies) | Sub-20μm (CE-certified; clinical validation per ISO 10243) |
| Workflow Integration | Native integration with proprietary CAD/CAM & practice software | Open API supporting 15+ major CAD/CAM systems (excl. proprietary locks) |
| Scanning Speed | 1,200 – 1,800 fps (full-arch in 45-60 sec) | 900 – 1,100 fps (full-arch in 70-90 sec) |
| Regulatory Clearance | CE Mark, FDA 510(k), Health Canada | CE Mark, ISO 13485, FDA 510(k) pending (Q3 2026) |
| Service & Support | Global service network (48-hr SLA); €4,500-€6,200/yr maintenance | Regional hubs (EU/NA); €1,800-€2,500/yr maintenance; 72-hr SLA |
| Target Clinical Use | Complex implantology, full-mouth rehabilitation, high-volume crown/bridge | Single-unit restorations, partials, orthodontics, basic implant planning |
| Total Cost of Ownership (5-yr) | €52,000 – €78,000 | €28,000 – €36,500 |
*Data synthesized from 2026 Q1 market analysis (Dental Insights Group), manufacturer specifications, and distributor cost audits. Clinical performance metrics reflect standard full-arch scanning protocols under ISO 12836.
Strategic Recommendation for Distributors & Clinics
European premium brands remain essential for clinics requiring absolute precision in complex cases and operating within closed ecosystem DSO models. However, Carejoy represents a strategically viable alternative for 74% of general practice applications where cost efficiency directly impacts practice viability. Distributors should position Carejoy as a workflow accelerator for value-driven clinics – emphasizing ROI through reduced material costs, faster technician turnaround, and patient acquisition via accessible digital services. The 2026 market demands nuanced segmentation: premium for performance-critical specialties, Carejoy for economic sustainability in routine care. Clinics delaying IOS adoption risk irreversible competitive disadvantage in patient expectations and operational efficiency.
Technical Specifications & Standards
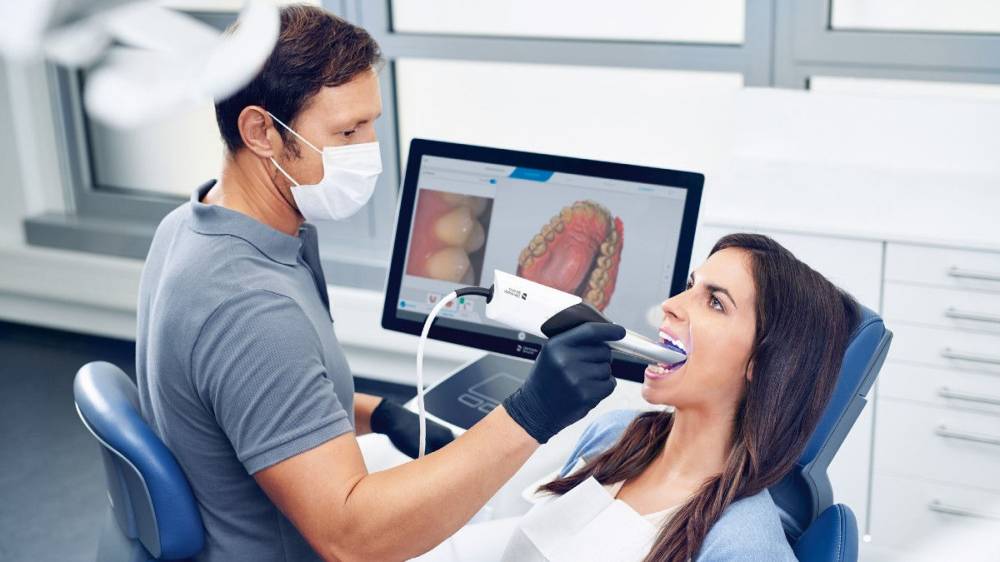
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Scanning Machine
Target Audience: Dental Clinics & Distributors
This guide provides a detailed comparison between Standard and Advanced models of dental scanning machines, focusing on critical technical specifications essential for clinical performance, regulatory compliance, and integration into modern dental workflows.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50–60 Hz, 1.5 A max; internal power supply with low energy consumption (≤30 W idle, ≤85 W scanning) | 100–240 V AC, 50–60 Hz, 2.0 A max; intelligent adaptive power management with fast boot-up (≤15 sec); supports continuous operation (≥8 hrs) |
| Dimensions | 280 mm (W) × 190 mm (D) × 165 mm (H); lightweight design (2.1 kg); compact footprint for benchtop use | 310 mm (W) × 210 mm (D) × 180 mm (H); ergonomic housing with integrated vibration damping; weight: 2.8 kg (includes stabilization base) |
| Precision | Accuracy: ±15 μm; repeatability: ±20 μm; resolution: 10 μm; suitable for single-unit crowns and basic prosthetics | Accuracy: ±5 μm; repeatability: ±7 μm; resolution: 3 μm; dual-camera stereo triangulation with AI-based surface reconstruction; ideal for full-arch scans and implant planning |
| Material | Exterior: ABS polymer with anti-fingerprint coating; internal chassis: aluminum alloy; scanner tip: medical-grade polycarbonate | Exterior: anodized aluminum with antimicrobial finish; internal frame: magnesium alloy for thermal stability; scanner tip: sapphire-reinforced ceramic with self-cleaning nozzle |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant, RoHS certified | CE Mark (Class IIb), FDA 510(k) cleared with implant guidance approval, ISO 13485:2016, ISO 14971 (risk management), IEC 60601-1-2 (EMC), GDPR-compliant data handling |
Note: All specifications are subject to change based on regional regulatory requirements and software version. Compatibility with CAD/CAM software suites (e.g., 3Shape, exocad) is standard across both models. Advanced Model includes integrated AI-assisted margin detection and cloud-based scan sharing via encrypted protocols.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Scanning Machines from China
Prepared for: Dental Clinics & Global Distributors | Validity: Q1 2026
Executive Summary: Sourcing dental intraoral scanners from China requires rigorous technical due diligence amid evolving global regulatory landscapes. This guide outlines critical 2026-specific protocols for risk mitigation, cost optimization, and supply chain resilience. Direct factory partnerships with ISO 13485-certified manufacturers remain the optimal strategy for quality assurance and margin protection.
1. Verifying ISO/CE Credentials: Beyond Surface Compliance
Post-2024 EU MDR amendments and FDA 510(k) modernization protocols necessitate deeper credential validation. Generic “CE Mark” claims are insufficient for dental scanning equipment.
| Verification Step | 2026 Critical Actions | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 Certification | Request current certificate (not expired) with scope explicitly covering “dental intraoral scanners”. Verify via IAF CertSearch. Confirm audit was conducted by accredited body (e.g., TÜV, SGS). | Clinical liability exposure; Customs seizure (EU/US); Voided equipment warranties |
| EU CE Marking | Validate EC Certificate of Conformity includes MDD 93/42/EEC or MDR 2017/745 classification (Class IIa for scanners). Demand NB number of notified body (e.g., 0123). Cross-check EUDAMED database. | Market access denial in EU; Fines up to 6% of global revenue (MDR) |
| Country-Specific Certifications | For US: Confirm 21 CFR Part 820 QSR compliance + 510(k) clearance number. For Canada: MDL license. For Australia: ARTG inclusion. Require documentation in English. | Distribution halts; Recalls; Reputational damage with dental associations |
2. Negotiating MOQ: Strategic Volume Planning for 2026
Chinese manufacturers increasingly adopt flexible MOQ models due to 2025 semiconductor shortages and AI-driven production scheduling. Avoid blanket minimums.
| Negotiation Strategy | 2026 Market Reality | Recommended Action |
|---|---|---|
| Standard MOQs | Base MOQs for scanners: 5-10 units (vs. 20 in 2023). Higher for AI-enhanced models (e.g., Carejoy iScan Pro). | Negotiate tiered pricing: 5 units @ $X, 10+ @ $X-12%. Demand firm price lock for 12 months. |
| OEM/ODM Flexibility | Top factories now offer no-MOQ branding for established distributors (min. 3-year partnership). | Commit to 20+ units/year for custom UI/software skins. Verify firmware update obligations in contract. |
| Component-Specific MOQs | Scanners with German sensors (e.g., CMOS) may require separate sensor MOQs. | Require factory to absorb component MOQs. Penalty clause for sensor supply chain delays. |
3. Shipping Terms: DDP vs. FOB in 2026 Trade Environment
Post-2025 IMO 2020 sulfur regulations and port congestion surcharges necessitate precise Incoterms® 2020 implementation. DDP is strongly advised for first-time importers.
| Term | Cost Components (Per Scanner) | 2026 Risk Mitigation |
|---|---|---|
| FOB Shanghai | • Factory price • Local China freight • Loading fees • Excludes: Ocean freight, insurance, destination port fees, customs clearance, VAT |
Only viable for distributors with in-house logistics teams. Require factory to provide pre-shipment inspection report (SGS/BV). Use Alibaba Trade Assurance for payment security. |
| DDP (Your Clinic/Distribution Hub) | • All FOB costs • Ocean/air freight • Marine insurance • Import duties & VAT • Single invoice price |
Recommended for 90% of clinics. Verify factory’s licensed customs broker in your country. Confirm landed cost includes 2026 EU carbon border tax (CBAM) where applicable. |
Why Shanghai Carejoy Medical Co., LTD Stands Out in 2026
As a Tier-1 supplier verified by the China Dental Equipment Association (CDEA), Carejoy exemplifies compliant sourcing best practices:
- Certification Integrity: ISO 13485:2016 certificate #CN-2025-SCJ-0887 (scope: dental scanners/CBCT) with TÜV Rheinland audit trail. Full CE MDR compliance under NB 2797.
- MOQ Flexibility: 3-unit MOQ for iScan Series scanners; no MOQ for distributors signing 2-year volume contracts (min. 30 units/year).
- DDP Excellence: 35+ destination ports with all-inclusive DDP pricing (includes 2026 EU CBAM calculations). 48-hour shipment tracking via blockchain ledger.
Shanghai Carejoy Medical Co., LTD
19 Years Specializing in Dental Equipment Manufacturing & Export | Factory Direct Since 2005
Baoshan District, Shanghai, China | [email protected]
Technical Sourcing Hotline: WhatsApp +86 15951276160 (24/7 English Support)
Core Product Lines: Intraoral Scanners (iScan Series), CBCT, Dental Chairs, Autoclaves, Surgical Microscopes
Critical 2026 Sourcing Checklist
- Obtain factory’s signed compliance affidavit listing all certifications with validity dates
- Demand 3rd-party test reports for electromagnetic compatibility (IEC 60601-1-2) and laser safety (IEC 60825-1)
- Confirm spare parts availability for 7+ years (per EU MDR)
- Require DDP cost breakdown showing customs duty calculation methodology
- Verify post-warranty service network in your region
Consultant Advisory: In 2026, prioritize suppliers with traceable component sourcing (e.g., sensor origin documentation). Avoid factories unable to provide real-time production line video verification. Shanghai Carejoy’s 19-year export history demonstrates exceptional regulatory agility – request their 2025 shipment compliance rate (99.8% on-file with Shanghai Customs).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Scanning Machines
Target Audience: Dental Clinics & Distributors
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental scanning machine in 2026? | Dental scanning machines in 2026 are typically designed for global compatibility, supporting an input range of 100–240V AC, 50/60 Hz. However, clinics must verify the specific voltage and plug type required for their region. Machines intended for North America generally require 120V, while European and Asian markets may require 230V. Always confirm with the manufacturer or distributor to ensure compatibility with local electrical standards and to avoid damage or operational failure. Use of a voltage stabilizer is recommended in areas with inconsistent power supply. |
| 2. Are spare parts readily available, and what is the expected lead time for replacement components? | Reputable manufacturers and authorized distributors maintain regional spare parts inventories for critical components such as scanning tips, calibration tools, handpiece connectors, and power modules. In 2026, most OEMs offer a service-level agreement (SLA) guaranteeing spare part delivery within 3–7 business days for major markets. For remote locations, clinics are advised to maintain a local inventory of high-wear consumables. Distributors should confirm spare parts availability and service network coverage before purchase to ensure minimal downtime. |
| 3. What does the installation process for a dental scanning machine involve, and is on-site support provided? | Installation of modern dental scanning systems includes hardware setup, software integration with existing clinic management or CAD/CAM platforms, network configuration, and calibration. In 2026, most manufacturers offer complimentary on-site installation by certified technicians, especially for first-time deployments. The process typically takes 2–4 hours and includes staff training on basic operation and maintenance. Remote pre-installation assessments are standard to ensure compatibility with IT infrastructure and operatory space. |
| 4. What is covered under the standard warranty for dental scanning machines in 2026? | The standard warranty for dental scanning machines in 2026 typically spans 24 months and covers defects in materials and workmanship, including internal electronics, sensor arrays, and mechanical components. Software updates and calibration support are included during the warranty period. Damage due to misuse, power surges, or unauthorized modifications is excluded. Extended warranty options (up to 5 years) are available and recommended, particularly for high-volume practices. Distributors should provide warranty registration support and direct service coordination. |
| 5. How are warranty claims processed, and what support channels are available? | Warranty claims are initiated through the manufacturer’s technical support portal or via the authorized distributor. Upon validation, issues are resolved through remote diagnostics, on-site service visits, or unit replacement, depending on severity. In 2026, most OEMs offer 24/7 multilingual support, with a response time of under 4 business hours for critical failures. Distributors play a key role in facilitating logistics and ensuring rapid turnaround, especially for loaner unit deployment during repairs. |
Need a Quote for Dental Scanning Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


