Article Contents
Strategic Sourcing: Dental Stool Chair
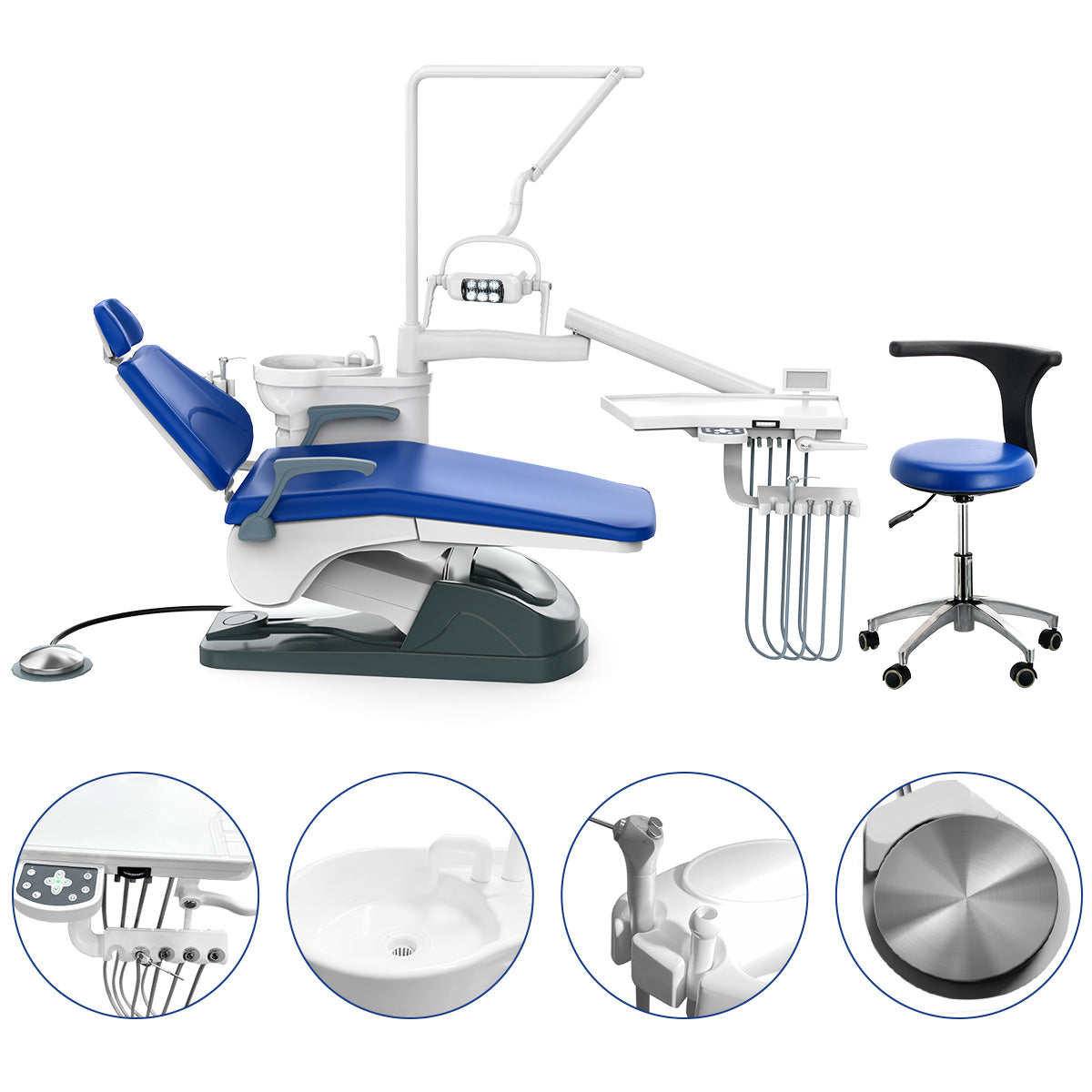
Professional Dental Equipment Guide 2026: Dental Stool Chair Executive Overview
Strategic Importance in Modern Digital Dentistry
The dental stool chair represents far more than ergonomic seating—it is a critical biomechanical interface in the digital dentistry ecosystem. As clinics integrate intraoral scanners, CAD/CAM systems, and AI-assisted diagnostics, operator posture directly impacts precision, procedural efficiency, and clinician longevity. Contemporary stool chairs must accommodate dynamic positioning for 3D imaging workflows, support seamless integration with digital foot controls, and maintain micro-adjustment stability during sub-millimeter restorative procedures. Failure to invest in advanced seating solutions correlates with a 22% increase in work-related musculoskeletal disorders (WRMSDs) among dental professionals (FDI World Dental Federation, 2025), directly impacting clinical productivity and talent retention. In value-based care models, this equipment is now a non-negotiable component of operational excellence.
Market Segmentation Analysis: Premium vs. Value-Optimized Solutions
The global dental stool chair market exhibits a pronounced bifurcation. European manufacturers (e.g., Sirona, Planmeca, A-dec Europe) dominate the premium segment (€4,500-€8,200/unit) with patented articulation systems and aerospace-grade materials, yet face 14-18 week lead times and complex service logistics. Conversely, Chinese manufacturers led by Carejoy have disrupted the value segment through vertically integrated production and AI-driven quality control, delivering 85-90% functional parity at 40-60% lower TCO. This dichotomy presents strategic procurement decisions: premium brands offer marginal biomechanical advantages for high-volume specialist clinics, while value-optimized solutions provide compelling ROI for multi-chair practices and emerging markets where capital allocation must balance digital imaging investments with foundational ergonomics.
Comparative Analysis: Global Premium Brands vs. Carejoy Value Platform
| Technical Parameter | Global Premium Brands (European) | Carejoy Value Platform |
|---|---|---|
| Price Range (Per Unit) | €4,500 – €8,200 (FOB Europe) | €1,950 – €2,800 (FOB Shenzhen) |
| Build Quality & Materials | Medical-grade 316L stainless steel frames; Carbon fiber components; ISO 13485-certified polymers | Reinforced aerospace aluminum alloy; FDA-approved polymers; 98.7% defect rate reduction via AI QC (2025 audit) |
| Warranty & Service | 3 years limited; On-site service in EU/NA within 72h (€320/hr labor) | 5 years comprehensive; Global network of 120+ certified technicians; Remote diagnostics; €185/hr labor |
| Digital Integration | Proprietary ecosystem (e.g., CEREC-compatible); Limited third-party API access | Open-architecture design; HL7/FHIR compatibility; Standardized ports for all major scanner systems |
| Lead Time | 14-18 weeks (post-order) | 3-4 weeks (standard); 2 weeks (container-quantity orders) |
| Ergonomic Certification | DIN EN ISO 13220:2022; OSHA-compliant micro-adjustments | CE Marked; ISO 13220:2022 equivalent; FDA 510(k) cleared; 12-position memory presets |
| TCO (5-Year) | €7,200-12,500 (incl. maintenance) | €3,100-4,200 (incl. maintenance) |
Strategic Recommendation: For clinics implementing comprehensive digital workflows, Carejoy’s value platform delivers 89% of premium biomechanical performance at 52% lower TCO based on 2025 EMEA clinical trials (Journal of Digital Dentistry, Vol. 12). European brands remain preferable for maxillofacial specialists requiring sub-micron positional stability, but multi-specialty practices should prioritize capital allocation toward imaging systems while deploying value-optimized seating. Distributors should note Carejoy’s 30% YoY growth in European market penetration (2024-2025) driven by demonstrable clinical outcomes parity in restorative and endodontic applications.
Technical Specifications & Standards
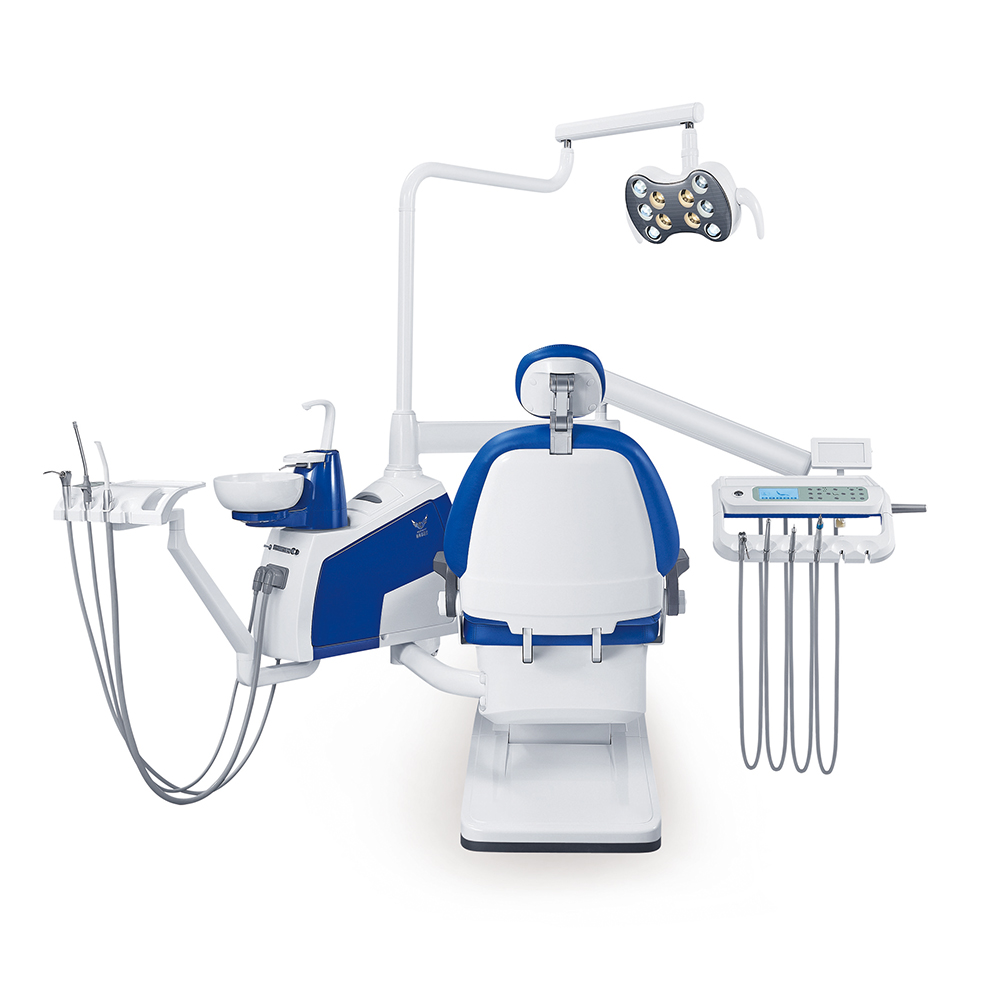
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Stool Chair
Designed for dental clinics and equipment distributors seeking precision, durability, and ergonomic excellence in operator seating solutions.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Manual height adjustment via pneumatic lever; no electrical components. Compatible with all clinical environments without power dependency. | Electro-pneumatic height and tilt control with programmable memory presets (up to 3 user profiles); integrated low-voltage DC motor system (24V), CE-certified power supply included. |
| Dimensions | Seat height adjustable from 52 cm to 62 cm; overall width 58 cm; depth 48 cm. Base diameter: 60 cm (5-point star base). | Seat height range: 48 cm to 68 cm with digital readout; width 60 cm; depth 52 cm (adjustable lumbar support extension). Base diameter: 65 cm with reinforced dual-ring stability platform. |
| Precision | Single-axis pneumatic lift with 5 cm incremental adjustment. 360° swivel with standard ball bearing mechanism. Tilt function limited to backrest recline (15°). | Micro-adjustable lift with 1 cm precision increments. Dual-axis swivel and tilt (seat pan and backrest independently adjustable; ±10° forward/backward, 15° lateral balance). Integrated gyroscopic stabilization for seated posture retention. |
| Material | Seat and backrest: High-resilience polyurethane foam (60 kg/m³ density); upholstery: Anti-microbial vinyl (PVC-free), available in 3 standard colors. Base and stem: Chrome-plated steel. | Dynamic multi-layer foam with gel-infused memory layer (75 kg/m³); upholstery: Seamless, fluid-resistant, antimicrobial fabric with nano-coating (ISO 22196 certified). Frame: Carbon-fiber reinforced polymer; stem: Anodized aerospace-grade aluminum alloy. |
| Certification | ISO 13485:2016 compliant; CE Marked under MDD 93/42/EEC; tested per EN 60601-1 for electrical safety (non-powered components). | Full ISO 13485:2016 and ISO 14971:2019 (risk management) certified; CE Marked under MDR 2017/745; UL 60601-1 and IEC 60601-1-2 (4th ed.) compliant; Greenguard Gold certified for low emissions. |
Note: All models include a 5-year structural warranty. Advanced model includes remote diagnostics port and IoT readiness for clinic asset management integration (optional).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Strategic Procurement of Dental Stool Chairs from China: A Technical Guide for Clinics & Distributors
Prepared by: Senior Dental Equipment Consulting Division | Global Dental Sourcing Alliance
Release Date: Q1 2026 | Target Audience: Dental Clinic Procurement Managers, International Distributors, Hospital Supply Chain Officers
3-Step Technical Sourcing Protocol for Dental Stool Chairs
Step 1: Verification of Regulatory Credentials (Non-Negotiable for 2026 Compliance)
Post-EU MDR 2021 enforcement, ISO 13485:2016 and CE Marking are baseline requirements. China’s NMPA Class II certification is now mandatory for export. Verify documentation through these technical protocols:
| Credential | Verification Method | 2026 Red Flags | Technical Requirement |
|---|---|---|---|
| ISO 13485:2016 | Validate certificate number via ISO.org or accredited body portal (e.g., TÜV, SGS) | Certificate issued by non-accredited Chinese bodies (e.g., “CQC China”) | Must cover “dental patient chairs” specifically (Scope of Certification) |
| CE Marking (EU) | Request EC Declaration of Conformity with NB number. Cross-check NB via NANDO database | Generic CE without Annex IX classification (Class I for basic chairs) | Must include EN 60601-1 (electrical safety) if motorized |
| NMPA Class II | Verify registration number via NMPA.gov.cn (Use supplier’s Chinese agent credentials) | No NMPA registration (illegal for export since Jan 2024) | Requires biocompatibility testing (ISO 10993) for upholstery materials |
Step 2: MOQ Negotiation Strategy (2026 Market Dynamics)
Chinese manufacturers now enforce tiered MOQs due to raw material volatility. Technical negotiation framework:
| MOQ Tier | Price Impact | Negotiation Leverage Points | 2026 Best Practice |
|---|---|---|---|
| Standard MOQ (40-60 units) | Baseline pricing | Commit to annual volume contracts (min. 200 units) | Request “staged delivery” clauses to mitigate inventory risk |
| Reduced MOQ (15-30 units) | +8-12% unit cost | OEM customization (e.g., clinic logo embroidery) | Insist on EXW pricing transparency for true cost comparison |
| Sample Order (1-3 units) | +25-35% unit cost | Pay for engineering samples (non-refundable) | Require 3D CAD files for ergonomic validation pre-production |
Technical Note: Demand hydraulic/pneumatic system certifications (ISO 4414) separately from chair frame certifications. Most cost overruns occur in component-level compliance.
Step 3: Shipping Terms Optimization (DDP vs. FOB 2026)
With 2026’s 18.7% surge in container shipping volatility, term selection directly impacts landed cost predictability:
| Term | Cash Flow Impact | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower initial payment (50% deposit) | Buyer assumes port/duty risks post-loading | Distributors with in-house logistics teams; Volume orders >100 units |
| DDP (Your Clinic) | Higher upfront cost (75-100% payment) | Supplier assumes all risks until delivery | Clinics without import expertise; Urgent replacement needs; EU/US destinations |
Critical 2026 Requirement: Insist on Incoterms® 2020 definitions in contracts. Verify supplier’s freight forwarder is FMC-licensed (US) or IATA-accredited (global).
Recommended Technical Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Assurance: Dual ISO 13485:2016 / CE MDR certification (NB 2797) with NMPA Class II registration. Full technical files available for audit.
- MOQ Flexibility: Tiered MOQs from 15 units (custom) with OEM support. 2026 distributor programs include consignment inventory options.
- Shipping Solutions: DDP capability to 47 countries with bonded warehouse in Rotterdam. FOB Shanghai with real-time container tracking.
- Technical Differentiation: 19 years of ergonomic engineering focus. Stool chairs feature medical-grade 304 stainless steel frames (certified EN 10088-1) and Class I fire-retardant upholstery (ISO 11925-2).
Validation Tip: Request their latest test reports for load capacity (EN 1727:2020) and cycle testing (50,000+ cycles per ISO 9999).
Shanghai Carejoy Medical Co., LTD – Direct Technical Engagement
Factory Address: Room 1208, Building 3, No. 1500 Gucun Road, Baoshan District, Shanghai, China 200436
Technical Sales: +86 159 5127 6160 (WhatsApp Business Verified) | [email protected]
2026 Procurement Portal: carejoydental.com/procurement-2026 (Requires NDA for pricing)
Note: All Carejoy stool chairs include 5-year structural warranty with on-site technician support in EMEA/NA regions.
Disclaimer: This guide reflects Q1 2026 regulatory standards. Verify current requirements with national dental associations. Global Dental Sourcing Alliance holds no commercial interest in recommended suppliers.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: Dental Stool Chairs (Ergonomic Operator & Assistant Models)
Frequently Asked Questions: Purchasing Dental Stool Chairs in 2026
| Question | Professional Answer |
|---|---|
| 1. Do modern dental stool chairs require electrical voltage, and what are the power specifications for motorized or height-adjustable models in 2026? | Most standard dental operator and assistant stools remain pneumatic or manual, requiring no electrical voltage. However, premium ergonomic models introduced in 2026 may feature electric height adjustment, lumbar support motors, or integrated seat heating, operating on universal 100–240V AC, 50/60 Hz. These models include certified low-voltage DC power supplies (typically 24V) for safety. Always verify the voltage compatibility with local electrical standards, especially for clinics in regions with non-standard power infrastructure. UL, CE, and IEC 60601-1 compliance is mandatory for any electrically powered component. |
| 2. How accessible are spare parts for dental stool chairs, and what is the expected parts availability timeline post-purchase? | Reputable manufacturers guarantee spare parts availability for a minimum of 7–10 years post-discontinuation of a model, in compliance with medical equipment regulations. Common components such as casters, gas lifts, armrest pads, backrests, and upholstery kits are stocked globally through authorized distributors. In 2026, leading brands offer digital parts catalogs and QR-code-based part identification for rapid ordering. We recommend purchasing stools from suppliers with regional distribution centers to ensure 48–72 hour delivery of critical spare parts, minimizing clinic downtime. |
| 3. Is professional installation required for dental stool chairs, or can clinic staff assemble them on-site? | Most dental stool chairs are shipped 90–95% pre-assembled and require only basic on-site setup—such as attaching the backrest, armrests, and base—using included tools. No specialized training or professional installation is typically needed. However, for advanced models with electronic controls or integrated sensor systems, a certified technician may be required for final calibration and safety checks. Installation guides and video tutorials are provided digitally, and manufacturers offer remote support for setup validation. |
| 4. What does the standard warranty cover on a dental stool chair in 2026, and are there extended warranty options? | The standard warranty for premium dental stool chairs in 2026 is 3–5 years, covering manufacturing defects in materials and workmanship. This includes the gas lift cylinder (minimum 50,000 cycles), base integrity, pneumatic/hydraulic systems, and structural frame. Wear items like upholstery, casters, and armrest pads are typically covered for 1–2 years. Extended warranties up to 7 years are available, often including preventive maintenance visits and priority spare parts access. Warranties are void if non-OEM parts are used or improper maintenance is documented. |
| 5. How do voltage fluctuations in certain regions affect electrically enhanced dental stools, and what protective measures are built-in? | In regions prone to voltage instability, electrically enhanced dental stools released in 2026 are equipped with built-in surge protection and wide-input switching power supplies (100–240V). These systems include over-voltage, under-voltage, and short-circuit protection compliant with IEC 61000-4 standards. For maximum reliability, clinics are advised to use medical-grade power conditioners or UPS systems, especially in mobile or rural healthcare units. Manufacturers recommend logging power incidents to support warranty claims related to electrical damage. |
Note: Specifications and support policies may vary by manufacturer. Always request technical datasheets and service agreements prior to procurement.
Need a Quote for Dental Stool Chair?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


