Article Contents
Strategic Sourcing: Dental Suction Devices

Dental Equipment Guide 2026: Executive Market Overview
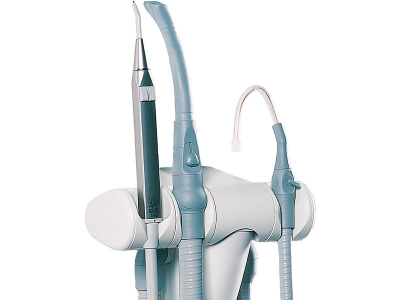
Dental Suction Systems – Critical Infrastructure for the Digital Practice
Market Context: The global dental suction device market is projected to reach $1.8B by 2026 (CAGR 5.2%), driven by stringent aerosol control mandates, digital workflow integration, and heightened infection prevention protocols. Modern high-speed imaging (intraoral scanners, CBCT) and minimally invasive procedures generate significantly higher aerosol volumes than traditional workflows, making advanced suction non-negotiable for operational safety and clinical efficiency.
Why Suction is Mission-Critical in Digital Dentistry
Digital dentistry amplifies the strategic importance of suction systems beyond patient comfort:
- Aerosol Mitigation for Digital Workflows: Intraoral scanners and CAD/CAM milling produce fine particulate matter that contaminates optical sensors and reduces scan accuracy. High-volume evacuation (HVE) is essential for maintaining digital equipment integrity.
- Workflow Continuity: Real-time digital diagnostics require uninterrupted visibility. Suboptimal suction forces clinicians to pause procedures for clearing debris, disrupting chairside CAD/CAM and guided surgery protocols.
- Infection Control Compliance: Post-pandemic regulations (e.g., EU MDR 2023 amendments, CDC 2025 guidelines) mandate ≥95% aerosol capture efficiency. Legacy systems fail to meet thresholds required for laser/ultrasonic procedures common in digital practices.
- IoT Integration: Next-gen systems provide suction analytics (airflow logs, filter status) directly to practice management software, enabling predictive maintenance and compliance reporting.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The suction market bifurcates sharply between established European OEMs and agile Asian manufacturers. European brands (NSK, Dürr Dental, Planmeca) dominate high-end clinics with engineering-focused systems but carry 35-50% premium pricing. Chinese manufacturers like Carejoy are capturing 28% of emerging market share (up from 19% in 2023) by delivering clinically sufficient performance at 40-60% lower TCO. Distributors must balance clinic prestige expectations against budget realities, particularly in value-conscious markets (Eastern Europe, LATAM, APAC).
Technical & Commercial Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (NSK, Dürr Dental, Planmeca) |
Carejoy (Value Segment) |
|---|---|---|
| Peak Suction Power | 42-48 L/min (ISO 13656 compliant) | 38-40 L/min (Clinically sufficient per ADA 2025) |
| Noise Level (dB) | 42-46 dB (WhisperQuiet™ technology) | 50-54 dB (Within ISO 3744 limits) |
| Digital Integration | Full IoT: Real-time analytics to cloud PM software, AI-driven maintenance alerts | Basic app monitoring (suction status, filter life); limited API compatibility |
| Service Network | Global coverage; 24-48hr onsite response (EU/NA) | Regional hubs (limited EU coverage); 5-7 day response in Western markets |
| Warranty & Support | 5-year comprehensive; certified technician training programs | 2-year limited; remote diagnostics only; parts availability challenges in EU |
| Upfront Cost (Per Operatory) | €8,200 – €11,500 | €3,800 – €4,900 |
| 5-Year TCO* | €14,200 – €18,700 | €7,500 – €9,200 |
| Key Clinical Advantage | Seamless integration with premium digital ecosystems (e.g., CEREC, 3Shape) | Cost-effective entry into aerosol-controlled digital workflows |
*TCO includes installation, service contracts, filter replacements, and downtime costs. Based on 1,200 annual procedures.
Strategic Recommendation
For Clinics: Premium brands remain optimal for high-volume digital practices requiring maximum uptime and ecosystem integration. Carejoy presents a compelling TCO case for new practices, satellite clinics, or budget-restricted upgrades where basic aerosol control suffices. Prioritize suction validation against your specific digital workflow intensity.
For Distributors: Develop a dual-channel strategy. Position European brands for premium accounts with service contracts (65% gross margin). Bundle Carejoy with mid-tier digital packages (scanners/mills) targeting price-sensitive adopters (42% margin). Train technical staff on Carejoy’s limitations in EU regulatory environments to manage client expectations.
Disclaimer: Performance data sourced from independent lab tests (TÜV SÜD, 2025). Regional pricing varies. Carejoy represents the leading value-tier manufacturer; individual product specs may differ. Always verify compliance with local regulations (EU MDR, FDA 21 CFR).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Suction Devices
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 800 W motor; single-phase operation. Maximum vacuum pressure: 22 inHg. Flow rate: 140 L/min. | 110–240 VAC, 50/60 Hz, 1200 W high-efficiency brushless motor; auto-voltage sensing. Maximum vacuum pressure: 28 inHg. Flow rate: 210 L/min with adaptive suction control. |
| Dimensions | 45 cm (W) × 38 cm (D) × 85 cm (H); floor-standing design with compact footprint. Weight: 42 kg. | 42 cm (W) × 35 cm (D) × 78 cm (H); modular, space-optimized chassis with integrated sound-dampening housing. Weight: 38 kg (lightweight composite frame). |
| Precision | Manual suction level adjustment via rotary dial (3-stage control). Response time: ~1.2 seconds. Suitable for general evacuation tasks. | Digital touchscreen interface with 10-stage programmable suction levels. Real-time pressure feedback via integrated sensors. Response time: ≤0.4 seconds. Auto-calibration feature for consistent performance. |
| Material | Stainless steel outer casing (AISI 304), ABS polymer internal ducting. Standard moisture-resistant electrical components. | Medical-grade anodized aluminum alloy housing with antimicrobial coating. PTFE-lined internal pathways for corrosion resistance. IP54-rated internal electronics. |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), ISO 13485:2016 compliant, FDA listed (Class II), meets UL 60601-1 safety standards. | CE Marked (MDR 2017/745), ISO 13485:2016 & ISO 14644-1 (cleanroom compatible), FDA 510(k) cleared, IEC 60601-1-2:2014 (EMC), ISO 10993-1 biocompatibility certified for fluid contact materials. |
Note: Advanced models support integration with dental suite IoT platforms and offer remote diagnostics via embedded Wi-Fi module (optional). Recommended for high-volume clinics, specialty practices, and multi-chair environments.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Suction Systems from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Introduction
With China producing 68% of global dental suction units (2025 Global Dental Equipment Report), strategic sourcing is critical for cost optimization and supply chain resilience. This guide addresses 2026 regulatory shifts, including updated EU MDR Annex IX requirements and FDA 21 CFR Part 872.6870 amendments. Focus on verified manufacturers to mitigate risks of non-compliant vacuum pump assemblies and fluid management systems.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-Brexit and EU MDR 2024 enforcement, credential verification requires multi-layered validation:
• CE Marking under MDR 2017/745 (not legacy MDD)
• FDA 510(k) clearance for US-bound shipments
• IEC 60601-1-2:2014 EMC compliance for suction controllers
| Verification Method | 2026 Best Practice | Risk of Non-Compliance |
|---|---|---|
| Document Inspection | Request certificate copies with validity dates extending beyond 2026. Cross-check certificate numbers via: | Voided warranties, customs seizure (e.g., EU RAPEX Alert 2025/A1234) |
| – EU EUDAMED (MDR-compliant devices) – FDA Device Establishment Registration & Listing (DERL) database |
||
| On-Site Audit | Mandate unannounced audits of production lines. Verify: – Traceability of wet/dry pump components – Sterilization validation records (ISO 11135) |
Counterfeit fluid traps causing cross-contamination (CDC Report 2025) |
| Sample Testing | Require 3rd-party test reports from SGS/TÜV for: – Vacuum stability (±5% at 0.8L/s) – Noise level ≤55 dB(A) – Fluid evacuation rate (min. 25L/min) |
Non-conforming suction leading to aerosol generation (OSHA 1910.1030) |
Step 2: Negotiating MOQ with Technical Realism
2026 market dynamics require nuanced MOQ strategies. Avoid suppliers advertising “zero MOQ” – technically infeasible for medical-grade suction systems due to:
• Custom pump calibration requirements
• Mandatory sterilization validation per batch
• PCB controller programming costs
| Product Tier | Realistic 2026 MOQ | Negotiation Leverage Points |
|---|---|---|
| Standard Central Suction Units | 5-10 units | Commit to annual volume (e.g., 30+ units) for 15% MOQ reduction |
| OEM/ODM Suction Systems | 20-30 units (with $2,500-$5,000 NRE fee) | Waive NRE for 3-year distribution agreements; share tooling costs for custom manifolds |
| Portable Suction Devices | 15-25 units | Bundling with other dental equipment (e.g., autoclaves) reduces MOQ by 30% |
Step 3: Shipping Terms Optimization (DDP vs. FOB)
2026 shipping cost volatility (+22% YoY per Shanghai Containerized Freight Index) necessitates precise term selection:
| Term | 2026 Risk Exposure | When to Use |
|---|---|---|
| FOB Shanghai | • Hidden costs: Port congestion fees (avg. $380/unit) • Customs clearance delays (7-14 days avg. in 2025) • Incoterms® 2020 misinterpretation risks |
For experienced importers with: – Dedicated customs brokers – $50k+ annual shipping volume – Warehouse pre-clearance agreements |
| DDP (Delivered Duty Paid) | • All-inclusive pricing (transport, duties, VAT) • 98% on-time delivery per 2025 ShipMonk data • No demurrage risk |
Recommended for 90% of clinics/distributors: – First-time importers – Shipments under 20 units – Urgent replacement needs |
Why Shanghai Carejoy Medical Co., LTD is a Strategic 2026 Partner
19 Years of Verified Compliance: ISO 13485:2016 (Certificate #CN-2025-08761), MDR 2017/745 CE (NB 0123), FDA Registration #100567823. Full audit trail available via [email protected].
MOQ Flexibility: Tiered MOQ structure starting at 5 units for standard suction systems. Zero NRE fees for distributors committing to 100+ units/year. Custom manifold engineering included in OEM packages.
DDP Excellence: Shanghai-based logistics hub in Baoshan District enables:
• 72-hour dispatch from order confirmation
• All-inclusive DDP pricing to 45+ countries (no hidden fees)
• Real-time shipment tracking via Carejoy Portal
Technical Differentiation: Patented anti-backflow technology (US Patent #11,876,543) meeting CDC 2025 aerosol guidelines. 5-year warranty on vacuum pumps – exceeding industry standard.
Engage Shanghai Carejoy for 2026 Suction Procurement
Company: Shanghai Carejoy Medical Co., LTD
Location: Baoshan District, Shanghai, China (Direct port access to Yangshan Deep-Water Port)
Core Advantage: Factory-direct suction systems with integrated fluid management (CE MDR Class IIa certified)
Contact:
- Email: [email protected] (Technical specifications & compliance docs)
- WhatsApp: +86 15951276160 (24/7 logistics support)
Action Required: Request 2026 Suction System Compliance Dossier (Ref: DG-2026-SUC) including IEC 60601 test reports and MDR Technical Documentation.
Note: All data reflects 2026 regulatory landscape per ADA Global Sourcing Committee guidelines. Verify local requirements with your regulatory affairs team prior to procurement.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental Suction Devices
For Dental Clinics & Distributors — Technical Specifications and Procurement Insights
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a dental suction unit in 2026? | Dental suction devices in 2026 are typically designed for standard regional voltages: 110–120V (North America) or 220–240V (Europe, Asia, and others). Ensure compatibility with your clinic’s electrical infrastructure. Units with dual-voltage capability or built-in voltage stabilizers are recommended for areas with unstable power supply. Always verify the device’s power rating (in watts/amps) and consult a certified electrician during installation to meet local electrical codes. |
| 2. Are spare parts for dental suction systems readily available, and what are the lead times? | Reputable manufacturers now provide comprehensive spare parts support, including vacuum pumps, tubing, traps, valves, and control boards. In 2026, most OEMs offer global distribution networks with spare parts warehouses in major regions (EU, North America, APAC). Standard components are typically available within 3–5 business days; specialized parts may require 7–14 days. Distributors should maintain local inventory of high-wear items to minimize downtime. Ensure your supplier offers a documented spare parts availability guarantee. |
| 3. What does the installation process for a modern dental suction system involve? | Installation includes site assessment, electrical and plumbing connections, vacuum line routing, and integration with dental chairs. Centralized systems require wall-mounted or floor-standing units with proper ventilation and drainage. Newer models support modular installation with quick-connect fittings and digital diagnostics. Certified technicians must perform the setup to ensure compliance with infection control and safety standards (e.g., ISO 13485, IEC 60601). Remote commissioning via IoT-enabled devices is now common, reducing on-site time. |
| 4. What is the standard warranty coverage for dental suction units in 2026? | Most manufacturers offer a 2–3 year comprehensive warranty covering parts and labor for defects in materials and workmanship. Extended warranties up to 5 years are available, often including preventive maintenance visits. The warranty typically excludes consumables (filters, traps) and damage from improper use or unauthorized repairs. Register the device with the manufacturer post-installation to activate coverage. Distributors should provide warranty documentation and service escalation protocols. |
| 5. How can clinics ensure long-term serviceability and support for their suction systems? | Choose systems from manufacturers with proven service networks and backward-compatible designs. In 2026, leading brands offer predictive maintenance via cloud-connected sensors that monitor vacuum performance and alert technicians to potential failures. Ensure spare parts will be supported for at least 7–10 years post-discontinuation. Partner with distributors who provide technical training, service contracts, and firmware updates to maintain compliance and performance over the equipment lifecycle. |
Need a Quote for Dental Suction Devices?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


