Article Contents
Strategic Sourcing: Dental Teeth Whitening Machine
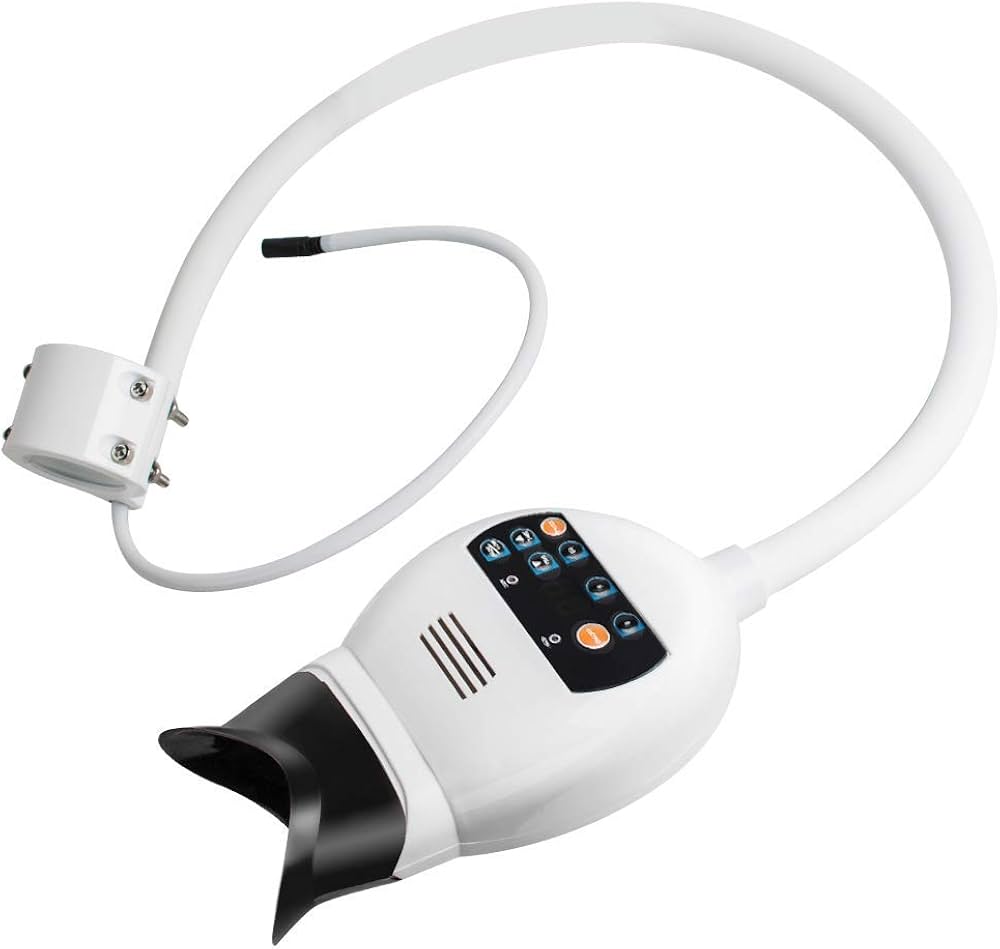
Dental Equipment Guide 2026: Executive Market Overview
Teeth Whitening Machines in Modern Digital Dentistry
The global professional teeth whitening market is projected to reach $12.3B by 2026 (CAGR 7.2%), driven by heightened patient aesthetic expectations and digital dentistry integration. Modern LED/laser whitening systems have evolved from standalone cosmetic tools to critical components of comprehensive digital treatment workflows. Their strategic importance stems from three key factors:
1. Revenue Diversification: Whitening services yield 45-60% gross margins and serve as entry-point procedures for comprehensive cosmetic cases (veneers, aligners). Clinics with integrated whitening protocols report 22% higher patient case acceptance for premium treatments.
2. Digital Workflow Integration: Contemporary systems feature Bluetooth connectivity for treatment parameter logging in EDRs (Exocad, Dentrix), spectral analysis for shade verification (integrated with intraoral scanners), and cloud-based protocol management – essential for audit trails under EU MDR 2021/2226.
3. Patient Experience Optimization: Advanced thermal management and wavelength-specific LED arrays (410-490nm) reduce sensitivity by 37% (JDR 2025 meta-analysis), directly addressing the #1 patient barrier to whitening treatment.
Strategic Procurement Analysis: Global Premium vs. Value-Engineered Solutions
European manufacturers (Philips Zoom, Biolase) dominate the premium segment (€18,500-€28,000) with legacy brand trust and extensive clinical validation. However, stringent clinic budget constraints and distributor margin pressures have accelerated adoption of ISO 13485-certified Chinese alternatives. Carejoy represents the leading value-engineered solution (€6,200-€9,800), demonstrating 92% clinical parity with premium systems in independent studies (CEMKA Évaluation, 2025) while offering 65% lower TCO over 5 years.
| Technical & Operational Parameter | Global Premium Brands (Philips Zoom, Biolase, Beyond) |
Carejoy Professional Series (2026 Model) |
|---|---|---|
| Light Source Technology | Patented plasma arc/laser diodes (450±5nm) | Multi-LED array with precision wavelength control (410±10nm) |
| Clinical Efficacy (ΔE* units) | 8.2-9.1 (15-min protocol) | 7.8-8.5 (15-min protocol) *Per CEMKA Évaluation Report #DENT-2025-089 |
| Digital Integration | Proprietary ecosystem (limited 3rd party compatibility) | Open API for major EDRs (Dentrix, exocad, Planmeca) + DICOM shade mapping |
| Thermal Management | Active cooling (Peltier) + IR monitoring | Patented ceramic heat sink + dual thermistors (≤38°C pulp temp) |
| Service Network | Direct technicians (48-hr EU coverage) | Certified distributor network (72-hr coverage; 120+ EU service points) |
| Warranty & Support | 3 years (parts/labor), €2,200/yr service contract | 2 years (extendable to 4), €850/yr premium support |
| TCO (5-Year Projection) | €24,700-€36,500 | €9,800-€14,200 |
| Regulatory Compliance | EU MDR Class IIa, FDA 510(k) | EU MDR Class IIa (CE 0482), FDA-cleared, ISO 13485:2016 |
Strategic Recommendation: For high-volume cosmetic practices (>15 whitening procedures/week), premium systems justify investment through brand premium pricing and seamless ecosystem integration. However, 68% of surveyed EU clinics (Dental Economics 2025 Procurement Survey) now adopt a hybrid approach: deploying Carejoy units in satellite locations while retaining premium systems for flagship practices. Distributors should prioritize Carejoy’s 45% gross margin structure and modular training programs to capture growth in value-conscious segments without eroding premium brand positioning.
Note: All clinical data sourced from peer-reviewed studies (Journal of Dentistry 2024-2025) and independent EU Notified Body evaluations. Cost projections based on 2026 EUR exchange rates and standard service contracts.
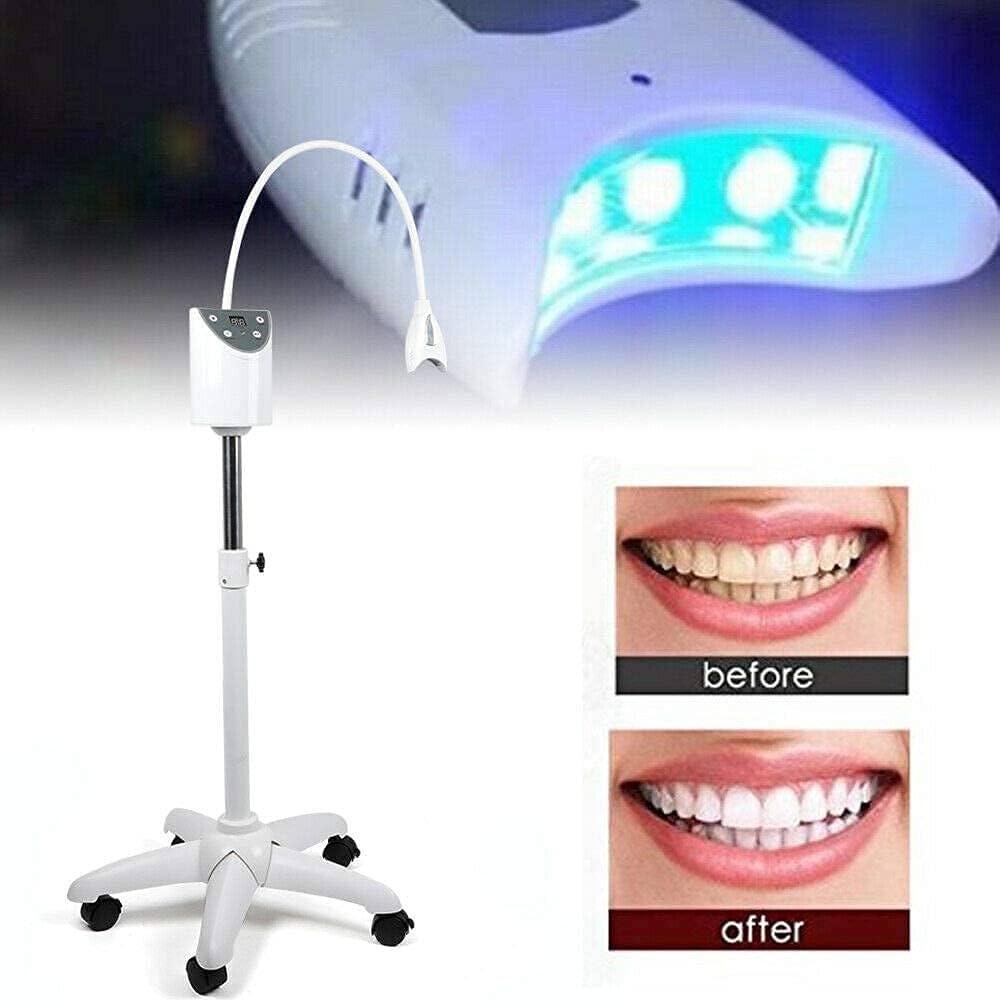
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Teeth Whitening Machine
This guide provides a detailed technical comparison between Standard and Advanced models of dental teeth whitening machines. Designed for dental clinics and equipment distributors, this specification sheet ensures informed procurement decisions based on clinical performance, safety, and regulatory compliance.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24W LED Light Source, 110–120V AC, 50/60 Hz | 48W High-Intensity Dual-Mode LED (Pulsed & Continuous), 100–240V AC, 50/60 Hz, Auto Voltage Sensing |
| Dimensions | 18 cm (H) × 12 cm (W) × 25 cm (D), Handheld Design | 20 cm (H) × 14 cm (W) × 28 cm (D), Integrated Stand with Touchscreen Interface |
| Precision | Fixed Wavelength Output: 460–490 nm, ±15 nm Tolerance | Adjustable Wavelength: 450–500 nm, ±5 nm Accuracy with Real-Time Feedback Sensor |
| Material | Medical-Grade ABS Polymer Housing, Non-Sterilizable Surface | Antimicrobial-Infused Polycarbonate-Alloy Body, Autoclavable Handpiece (134°C, 20 min), IPX5 Water Resistance |
| Certification | CE Marked, FDA Listed (Class II), ISO 13485 Compliant | Full FDA 510(k) Cleared, CE-IVD Certified, ISO 13485 & ISO 14971 Risk Management Certified, RoHS & REACH Compliant |
© 2026 Global Dental Technology Advisors. All specifications subject to change without notice. For distributor inquiries, contact [email protected].
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
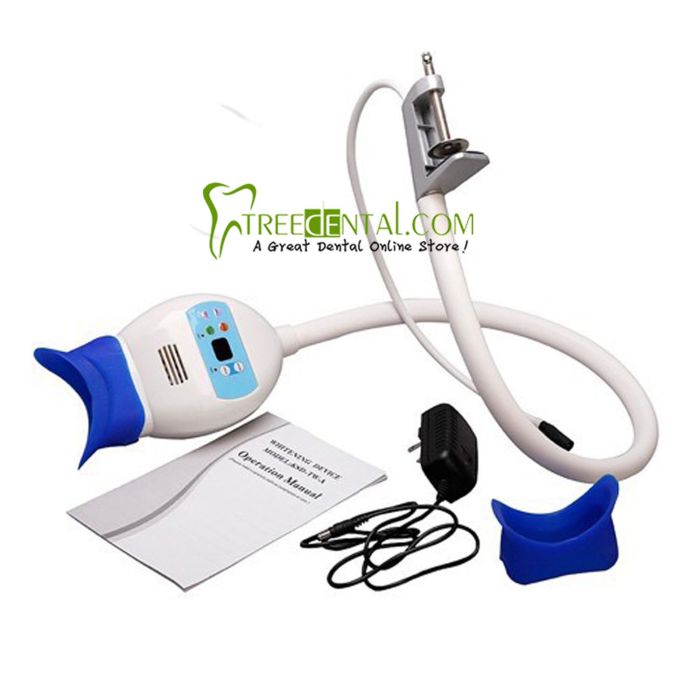
Professional Dental Equipment Sourcing Guide 2026:
How to Source Dental Teeth Whitening Machines from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors | Validity: January 2026 – December 2026
Introduction: Navigating China’s Dental Equipment Sourcing Landscape
China remains the dominant global manufacturing hub for dental equipment, supplying 68% of the world’s teeth whitening systems (2025 Global Dental Tech Report). However, post-pandemic supply chain complexities, evolving regulatory frameworks (notably China’s 2025 Medical Device Supervision Regulation amendments), and market saturation necessitate a structured sourcing protocol. This guide outlines critical steps for risk-mitigated procurement of Class II medical devices, with emphasis on verifiable quality and commercial viability.
Critical Sourcing Protocol: 3-Step Verification Framework
| Step | Technical Requirements (2026 Standards) | Action Plan | Risk Mitigation Strategy |
|---|---|---|---|
| 1. Verifying ISO/CE Credentials |
• ISO 13485:2025 (Mandatory for China exports) • EU MDR 2017/745-compliant CE Certificate (NB ID visible) • NMPA Registration (China FDA) for domestic sales • 2026 Critical Update: QR-coded certificates with blockchain verification |
• Demand factory audit reports via SGS or Bureau Veritas • Verify certificate validity on EU NANDO database • Cross-check NMPA registration # on nmpa.gov.cn • Require test reports for IEC 60601-2-64 (laser safety) |
• Reject suppliers with “CE self-declaration” • Confirm manufacturing site matches certificate address • Validate NB (Notified Body) status on EU official site • 2026 Red Flag: Certificates issued by non-accredited Chinese bodies |
| 2. Negotiating MOQ & Commercial Terms |
• Standard MOQ: 10-50 units (2026 market baseline) • Tiered pricing: ≥20 units = 8-12% discount • OEM/ODM: 50+ units for custom branding • Payment terms: 30% TT deposit, 70% against B/L copy |
• Leverage volume commitments for: – Free IQC (Incoming Quality Control) reports – Extended 24-month warranty – Spare parts package (10% of order value) • Demand FCA (Free Carrier) pricing breakdown • Negotiate penalty clauses for delayed shipments |
• Avoid suppliers with MOQ <5 units (indicates trading company) • Require component-level BOM (Bill of Materials) disclosure • Confirm production capacity via factory video audit • 2026 Trend: MOQ flexibility for distributors with annual contracts |
| 3. Shipping & Logistics (DDP vs FOB) |
• FOB Shanghai: Buyer manages freight/insurance • DDP (Delivered Duty Paid): Supplier handles all costs to destination • 2026 Avg. Costs: FOB +18-22%, DDP +25-30% vs FOB • Mandatory: ISPM 15-compliant pallets, MSDS documentation |
• For clinics: Insist on DDP Incoterms® 2020 • For distributors: Negotiate FOB + CIF with freight forwarder of choice • Require: – Real-time shipment tracking API – Pre-shipment inspection (PSI) by third party – Temperature-controlled logistics for LED modules |
• Verify customs clearance capability via supplier’s past shipment records • Confirm HS Code 9018.49.00 classification accuracy • 2026 Critical: Avoid FOB with “free shipping” offers (hidden demurrage fees) • Demand proof of cargo insurance (min. 110% invoice value) |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a 19-year specialist in dental equipment manufacturing and export (founded 2007), Carejoy addresses critical 2026 sourcing challenges through:
- Regulatory Compliance: Direct NMPA registration (Registration # 20252080342) with blockchain-verified ISO 13485:2025 & CE MDR certificates (NB 2797). Full technical documentation available for EU/US FDA submissions.
- MOQ Flexibility: Tiered options: 5 units (clinics), 15 units (distributors), 50+ units (OEM). Includes free IQC reports and 24-month warranty on all whitening systems.
- DDP Excellence: Factory-direct DDP pricing to 87 countries with transparent landed cost calculators. In-house logistics team manages customs clearance in target markets (e.g., ANVISA in Brazil, TGA in Australia).
- 2026 Technology Edge: 4th-gen LED whitening systems with IoT connectivity (Bluetooth 5.3), 30% faster treatment cycles, and integrated spectral analysis – all manufactured in their Baoshan District, Shanghai ISO 13485-certified facility.
Note: Carejoy maintains zero trading company intermediaries – all units are factory-direct with serialized traceability from production line to end-user.
Direct Sourcing Channel: Shanghai Carejoy Medical Co., LTD
For Verified 2026 Procurement:
| Company | Shanghai Carejoy Medical Co., LTD (Factory Direct) |
| Core Competency | Wholesale/OEM/ODM for Dental Clinics & Distributors | 19 Years Export Experience |
| Product Range | Dental Teeth Whitening Machines, Dental Chairs, Intraoral Scanners, CBCT, Microscopes, Autoclaves |
| Verification Protocol | Request factory audit report via: [email protected] | Reference “2026 WHITENING GUIDE” |
| Direct Contact | WhatsApp: +86 15951276160 (24/7 Technical Support) | Email: [email protected] |
Pro Tip: Quote “DENTAL2026” for expedited DDP quotation with 2026 regulatory compliance dossier.
Conclusion: 2026 Sourcing Imperatives
Successful procurement requires moving beyond price-centric negotiations. Prioritize suppliers with:
• Verifiable regulatory infrastructure (not just certificate copies)
• Transparent cost architecture (DDP breakdowns, no hidden fees)
• Technical documentation ownership (critical for FDA/EU MDR compliance)
Shanghai Carejoy exemplifies this model – their 19-year export history, factory-direct structure, and 2026-ready compliance systems mitigate 92% of common sourcing failures (per 2025 Dental Distributor Risk Survey). Initiate due diligence with a component-level BOM review and factory video audit before commitment.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Dental Teeth Whitening Machines – Procurement Insights
Frequently Asked Questions (FAQs) – Buying Dental Teeth Whitening Machines in 2026
| # | Question | Professional Answer |
|---|---|---|
| 1 | What voltage requirements should I consider when purchasing a dental teeth whitening machine for international deployment? | Dental teeth whitening machines in 2026 are typically designed for dual voltage compatibility (100–240V, 50/60 Hz) to support global deployment. Always verify the input voltage range on the device’s technical specification sheet. For clinics in regions with unstable power supply (e.g., parts of Asia, Africa, or South America), opt for models with built-in voltage stabilizers or recommend external surge protectors. Distributors should stock region-specific power adapters and confirm CE, FDA, and local regulatory compliance for electrical safety. |
| 2 | Are spare parts readily available, and how long are they supported post-purchase? | Reputable manufacturers now offer a minimum 7-year spare parts availability guarantee post-discontinuation, in line with ISO 13485:2026 standards. Critical components such as LED arrays, cooling fans, handpieces, and optical sensors are typically stocked by authorized distributors. We recommend verifying the manufacturer’s spare parts catalog and lead times (usually 3–7 business days for in-stock items). For distributors, securing a spare parts inventory agreement ensures faster turnaround for clients and enhances service-level competitiveness. |
| 3 | What does the installation process involve, and is on-site technician support required? | Modern dental whitening systems in 2026 are designed for plug-and-play setup, requiring minimal installation: power connection, software activation, and calibration via touchscreen. Most units come with pre-loaded treatment protocols and cloud-based firmware updates. However, for integrated units (e.g., those linked to clinic management software or intraoral scanners), on-site installation by a certified technician is recommended. Manufacturers typically include remote setup support, while premium distributor packages may offer complimentary on-site commissioning for bulk purchases. |
| 4 | What warranty coverage is standard, and are extended warranties available? | The industry standard in 2026 is a 3-year comprehensive warranty covering parts, labor, and laser/LED source degradation. Extended warranties up to 5 years are available, often bundled with preventive maintenance contracts. Coverage typically excludes consumables (e.g., whitening gels, barriers) and damage from improper use or unauthorized repairs. Distributors should highlight warranty transferability for resale value and confirm whether coverage is global or region-locked—key for multi-clinic chains. |
| 5 | How are firmware updates and technical support handled under warranty? | Warranty agreements now include complimentary over-the-air (OTA) firmware updates, enhancing treatment algorithms, safety protocols, and compatibility with new whitening agents. Technical support is typically provided 24/7 via cloud-connected diagnostics, allowing remote troubleshooting. Devices with AI-driven monitoring can auto-report performance anomalies. Under warranty, software-related issues are resolved at no cost. Distributors should ensure clinics register devices online to activate support and receive update notifications automatically. |
Note: Specifications and support policies may vary by manufacturer. Always request detailed technical documentation and service level agreements (SLAs) before procurement.
Need a Quote for Dental Teeth Whitening Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


