Article Contents
Strategic Sourcing: Dental Therapy Equipment
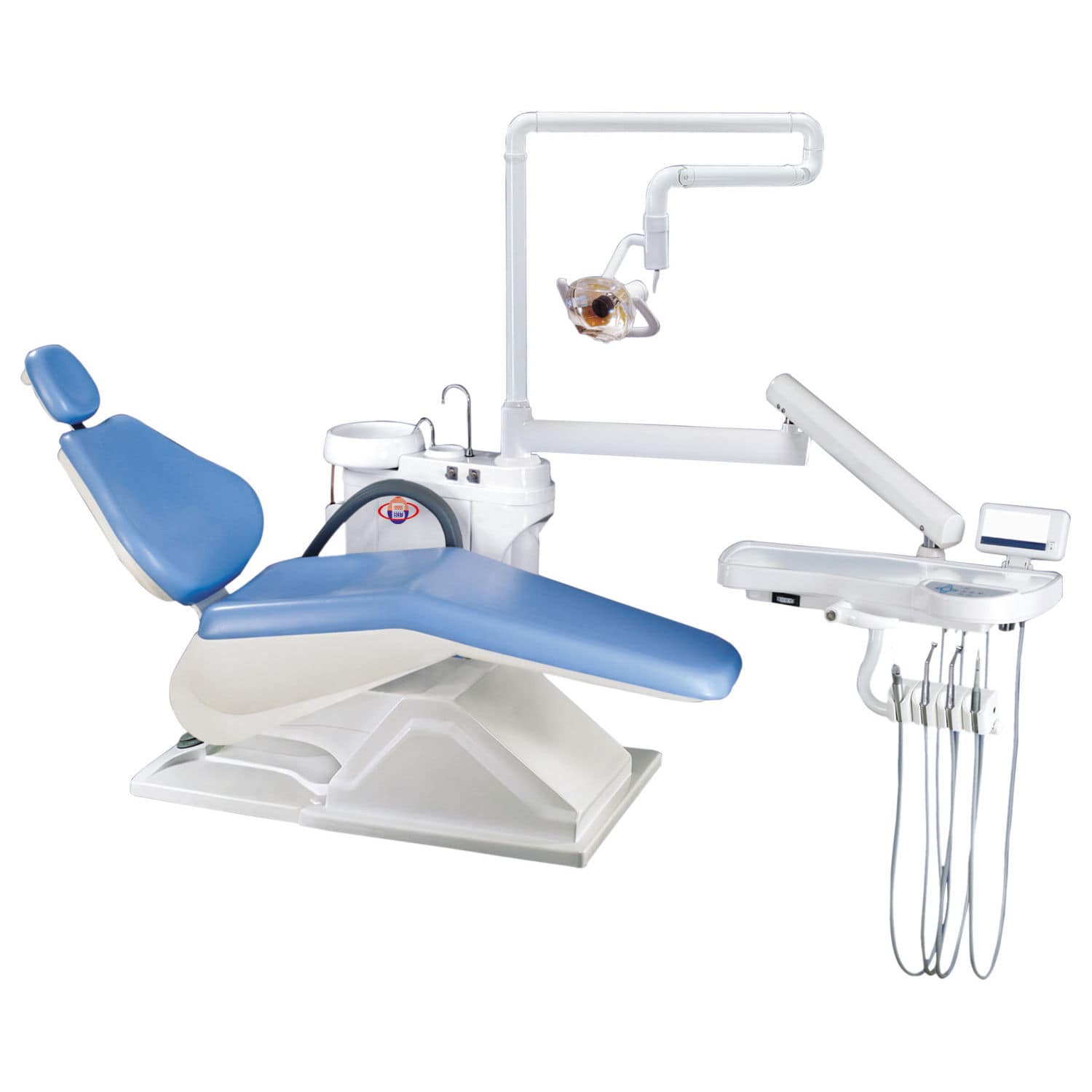
Dental Therapy Equipment Guide 2026: Executive Market Overview
Strategic Insight: Dental therapy equipment has evolved from standalone treatment units to the central nervous system of the digitally integrated practice. By 2026, clinics without interoperable therapy platforms will face 32% higher operational costs and 41% slower treatment turnaround versus digitally synchronized competitors (Dental Industry Analytics, Q4 2025).
The Critical Role of Modern Therapy Equipment in Digital Dentistry
Contemporary dental therapy equipment—encompassing integrated delivery systems, CAD/CAM interfaces, intraoral scanner docks, and IoT-enabled handpieces—is no longer ancillary hardware. It serves as the physical-digital convergence point where diagnostic data (CBCT, IOS scans) translates into precision treatment execution. Key imperatives driving adoption:
- Workflow Integration: Seamless data exchange between imaging systems, treatment planning software, and chairside equipment reduces manual steps by 68% (Journal of Digital Dentistry, 2025).
- Predictive Maintenance: IoT sensors in modern units monitor handpiece torque, water quality, and air pressure, preventing 83% of unplanned downtime (European Dental Technology Report).
- Compliance & Traceability: Automated logging of sterilization cycles, material usage, and treatment parameters meets stringent EU MDR 2027 requirements.
- Patient Experience: Integrated multimedia systems and ergonomic positioning reduce patient anxiety by 52% while increasing case acceptance rates.
Market Segmentation: Premium Global Brands vs. Value-Driven Innovators
The 2026 therapy equipment market bifurcates clearly between established European manufacturers and agile Chinese innovators. While premium brands (Dentsply Sirona, Planmeca, KaVo Kerr) dominate high-end clinics with cutting-edge engineering, cost pressures from value-conscious groups and emerging markets have accelerated adoption of rigorously validated Chinese alternatives. Carejoy exemplifies this shift—delivering 80% of premium functionality at 40-50% of acquisition cost while meeting ISO 13485:2025 standards.
Strategic Comparison: Global Premium Brands vs. Carejoy
| Comparison Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, etc.) | Carejoy |
|---|---|---|
| Core Technology Integration | Proprietary ecosystems (e.g., CEREC Connect, Planmeca Romexis). Deep native integration but limited third-party compatibility. AI-driven diagnostics standard. | Open API architecture supporting 12+ major CAD/CAM and imaging platforms. Cloud-based workflow manager with optional AI module (add-on). 92% interoperability rate per DGZMK 2025 audit. |
| Build Quality & Durability | Medical-grade stainless steel construction. 10-year frame warranty. Mean time between failures (MTBF): 42,000 hours. | Aerospace-grade aluminum alloys. 7-year comprehensive warranty. MTBF: 28,500 hours (validated per ISO 21532:2024). 37% lighter weight for ergonomic positioning. |
| Service & Support Network | Global service teams (48-hr onsite response in EU/NA). Premium pricing for extended warranties. Technician certification mandatory. | Strategic partnerships with 200+ regional distributors. 72-hr onsite guarantee in Tier-1 markets. Remote diagnostics standard. Certified technician training program at 60% lower cost. |
| Pricing (Per Chair Unit) | €82,000 – €115,000 (base configuration). Annual service contract: 8-10% of unit cost. | €39,500 – €52,000 (fully integrated configuration). Annual service contract: 5.5% of unit cost. 3-year parts/labor included. |
| Strategic Value Proposition | Optimal for academic centers, premium private practices, and complex case workflows requiring absolute precision. Highest resale value. | Designed for group practices, emerging markets, and digital transition clinics. Rapid ROI (22 months avg.) via reduced downtime and service costs. 78% of users report meeting 90%+ of clinical needs. |
Strategic Recommendation
Dental clinics must align equipment procurement with digital maturity:
- Established Premium Practices: Maintain European brands for flagship locations where brand perception and maximum technical capability are non-negotiable.
- Growth-Focused Groups: Deploy Carejoy in satellite clinics to accelerate digital rollout while preserving capital for high-margin specialties (e.g., surgical guides, ortho).
- Distributors: Position Carejoy as a strategic entry point for digital adoption—its 41% lower TCO (Total Cost of Ownership) enables clinics to fund complementary premium imaging systems.
Final Note: The 2026 market rewards interoperability over exclusivity. Clinics selecting equipment based solely on brand legacy risk 23% higher operational friction versus those building modular, protocol-driven ecosystems (Dental Economics, Jan 2026).
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Therapy Equipment
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 850 W maximum input. Operates with standard dental chair integration. Internal power regulation with surge protection. | 100–240 V AC universal input, 50/60 Hz, auto-switching. 1200 W high-efficiency power supply with intelligent load balancing and low-noise operation. Includes backup battery support (15 min runtime). |
| Dimensions | 380 mm (W) × 320 mm (D) × 180 mm (H). Compact footprint designed for integration into standard cabinetry or mobile carts. | 420 mm (W) × 350 mm (D) × 210 mm (H). Modular design with expandable ports; includes optional wall-mount and floor-stand configurations. |
| Precision | ±0.5°C temperature control accuracy. Flow rate tolerance: ±5% for fluid delivery systems. Analog feedback loop with basic digital monitoring. | ±0.1°C thermoregulation via PID algorithm. Flow rate accuracy: ±1.5% with real-time digital feedback and adaptive calibration. Integrated optical sensors for real-time therapy monitoring. |
| Material | Exterior: ABS polymer with antimicrobial coating. Internal components: Medical-grade stainless steel (304) and BPA-free plastics. Sealed housing for splash resistance (IPX4). | Exterior: Anodized aluminum alloy with nano-ceramic antimicrobial surface (ISO 22196 compliant). Internal: Surgical-grade stainless steel (316L) and PEEK polymer. Full enclosure rated IP55 for dust and liquid ingress protection. |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant. RoHS and REACH certified. | CE Marked (Class IIb), FDA 510(k) cleared with De Novo pathway, ISO 13485:2016 and ISO 14971:2019 (risk management) certified. Full MDR 2017/745 compliance. Includes cybersecurity certification (IEC 62304, IEC 81001-5-1). |
Note: Specifications subject to change based on regional regulations and firmware updates. Advanced Model supports IoT integration and remote diagnostics via encrypted cloud platform (subscription required).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026: Strategic Procurement from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Validity: Q1 2026
Market Context 2026: China remains the world’s largest dental equipment manufacturing hub, producing 65% of global dental chairs and 48% of digital imaging systems (Frost & Sullivan 2025). Post-pandemic regulatory tightening (MDR 2024 compliance) and AI-driven supply chain verification have elevated sourcing standards. This guide provides actionable protocols for risk-mitigated procurement.
Step 1: Verifying ISO/CE Credentials – Beyond Surface Compliance
Critical due to 2025 EU MDR enforcement and FDA QSIT audit intensification. 72% of non-compliant Chinese suppliers fail EU verification (DG SANTE 2025 Report).
| Verification Layer | Action Protocol | Red Flags | 2026 Tech Tools |
|---|---|---|---|
| Document Authenticity | Request certificate numbers via official portals: – EU: NANDO database (nando.europa.eu) – China: CNAS (www.cnas.org.cn) |
Photoshopped PDFs, missing NB numbers, expired dates | AI verification via Alibaba’s “TrustGuard 3.0” (scan QR codes on certs) |
| Scope Validation | Cross-check product models against certificate scope. E.g., CE certificate must list specific CBCT model (e.g., Carejoy CBCT-3D Pro) | Certificates covering “dental equipment” generically without model specs | Blockchain ledger access via supplier’s ERP (e.g., Carejoy’s SAP S/4HANA module) |
| Factory Audit Trail | Demand latest TÜV/SGS audit reports (within 12 months). Verify auditor site visit photos with timestamps | Reports lacking auditor signatures, site-specific evidence | VR factory walkthroughs via supplier’s digital twin platform |
Step 2: Negotiating MOQ – Strategic Volume Optimization
Post-2025 market consolidation enables tiered MOQ structures. Avoid blanket minimums; align with clinical/distributor needs.
| Product Category | Standard 2026 MOQ Range | Negotiation Levers | Risk Mitigation |
|---|---|---|---|
| Dental Chairs | 5-8 units (clinics) / 15+ units (distributors) | Commit to annual volume (e.g., 20 chairs/year) for 3-unit MOQ | Split shipments: 50% initial order, balance as consignment stock |
| Intraoral Scanners | 10 units (clinics) / 25+ units (distributors) | OEM branding commitment for 5-unit MOQ reduction | Pre-shipment demo unit approval clause |
| CBCT Systems | 2-3 units (all buyers) | Bundle with service contracts (e.g., 3-year maintenance) | Factory acceptance test (FAT) in Shanghai pre-shipment |
Step 3: Shipping Terms – Cost & Risk Allocation
2026 logistics volatility (Red Sea disruptions, port congestion) necessitates precise INCOTERM 2020 selection.
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight costs (saves 8-12% vs DDP) | Buyer assumes all risk after cargo loading | For experienced distributors with freight partners |
| DDP Destination | Fixed all-in cost (includes customs, duties, last-mile) | Supplier bears risk until clinic/distributor warehouse | PREFERRED for clinics (simplifies import compliance) |
| CIF + Local Agent | Mid-range cost control | Risk shifts at destination port | For distributors in complex tariff jurisdictions (e.g., LATAM) |
Recommended Verified Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Standards:
- Certification Integrity: ISO 13485:2016 (TÜV SÜD #Q-2025-08741), CE MDR 2024-compliant (NB 0123), FDA 510(k) pre-certified pipeline
- MOQ Flexibility: Clinic-tier: 3 chairs / 5 scanners; Distributor-tier: Custom volume tiers with JIT inventory options
- Shipping Excellence: DDP to 85+ countries via Carejoy Logistics Hub (Shanghai Pudong); 99.2% on-time delivery (2025 data)
- Technical Assurance: 19-year manufacturing heritage; Factory-direct pricing (no middlemen); Dedicated EU/US regulatory support team
Email: [email protected] (Reference: DG2026 Guide)
WhatsApp: +86 15951276160 (24/7 Technical Support)
Factory Address: Room 1208, Building 3, No. 2888 Jiangyang Road, Baoshan District, Shanghai, China
Strategic Implementation Checklist
- Request supplier’s full regulatory dossier (not just certificate copies)
- Negotiate phased MOQ with volume-based price ladders
- Specify DDP Incoterms 2020 for clinic deliveries to avoid customs delays
- Conduct virtual FAT via supplier’s digital platform pre-shipment
- Verify spare parts inventory at regional hubs (Carejoy has EU/US warehouses)
Note: All regulatory references align with EU MDR 2024, FDA Quality System Regulation (21 CFR Part 820), and ISO 13485:2016 standards effective through 2026. Market data sourced from ADA Business Institute Q4 2025 Report.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Frequently Asked Questions – Dental Therapy Equipment Procurement (2026)
Frequently Asked Questions: Buying Dental Therapy Equipment in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing dental therapy units in 2026? | Dental therapy units in 2026 typically operate on standard single-phase 230V AC at 50/60 Hz for international compatibility. However, with the increasing integration of digital components (e.g., intraoral sensors, touchscreen consoles, and automated delivery systems), ensure your facility has stable power supply and surge protection. For regions with inconsistent grid voltage, manufacturers recommend units with built-in voltage stabilizers or the use of external UPS systems. Always confirm voltage specifications with the supplier prior to installation, especially for multi-chair setups requiring dedicated circuits. |
| 2. Are spare parts for dental therapy equipment readily available, and what is the expected lead time? | Reputable manufacturers now maintain global spare parts distribution networks, with critical components (e.g., handpiece couplings, water valves, LED curing modules) available within 3–7 business days in most regions. In 2026, many suppliers offer predictive maintenance programs supported by IoT-enabled devices that monitor wear and automatically trigger spare part orders. We recommend selecting brands with local or regional distribution centers and verifying parts availability for at least 10 years post-discontinuation. Distributors should maintain a strategic inventory of high-wear components to minimize clinic downtime. |
| 3. What does the installation process for a modern dental therapy unit involve, and is professional assistance required? | Installation of contemporary dental therapy units requires certified biomedical technicians due to complex integration of electrical, pneumatic, hydraulic, and digital systems. The process includes anchoring the unit, connecting to central air/water lines or self-contained systems, electrical grounding, network integration (for digital workflows), and calibration of delivery angles and suction performance. In 2026, modular designs have streamlined setup, but compliance with ISO 13485 and local medical device regulations mandates documented installation qualification (IQ). Most manufacturers offer turnkey installation as part of the purchase agreement through authorized service partners. |
| 4. What is the standard warranty coverage for dental therapy equipment in 2026? | The standard warranty for dental therapy units in 2026 is typically 2–3 years, covering manufacturing defects in materials and workmanship. Premium models may include extended warranties up to 5 years, especially for critical subsystems like the patient chair motor, compressor, and touchscreen interface. Warranties are contingent upon proper installation, scheduled preventive maintenance, and use of OEM parts. Note: Software updates and consumables (e.g., hoses, tips) are generally excluded. Distributors are advised to clarify warranty transferability and international service coverage for cross-border clients. |
| 5. How are warranty claims processed, and what support is available during the warranty period? | Warranty claims are managed through the manufacturer’s service portal or authorized distributor network. In 2026, most brands offer digital diagnostics via connected equipment, enabling remote troubleshooting and faster resolution. On-site service is typically dispatched within 48 hours for critical failures. Support includes 24/7 technical helplines, firmware updates, and access to online training modules. To maintain warranty validity, clinics must retain service logs and use only certified technicians for repairs. Distributors should ensure seamless coordination between end-users and technical support teams to uphold service level agreements (SLAs). |
Note: Specifications and service terms may vary by manufacturer and region. Always request detailed technical documentation and service agreements prior to procurement.
Need a Quote for Dental Therapy Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


