Article Contents
Strategic Sourcing: Dental Turbine Unit
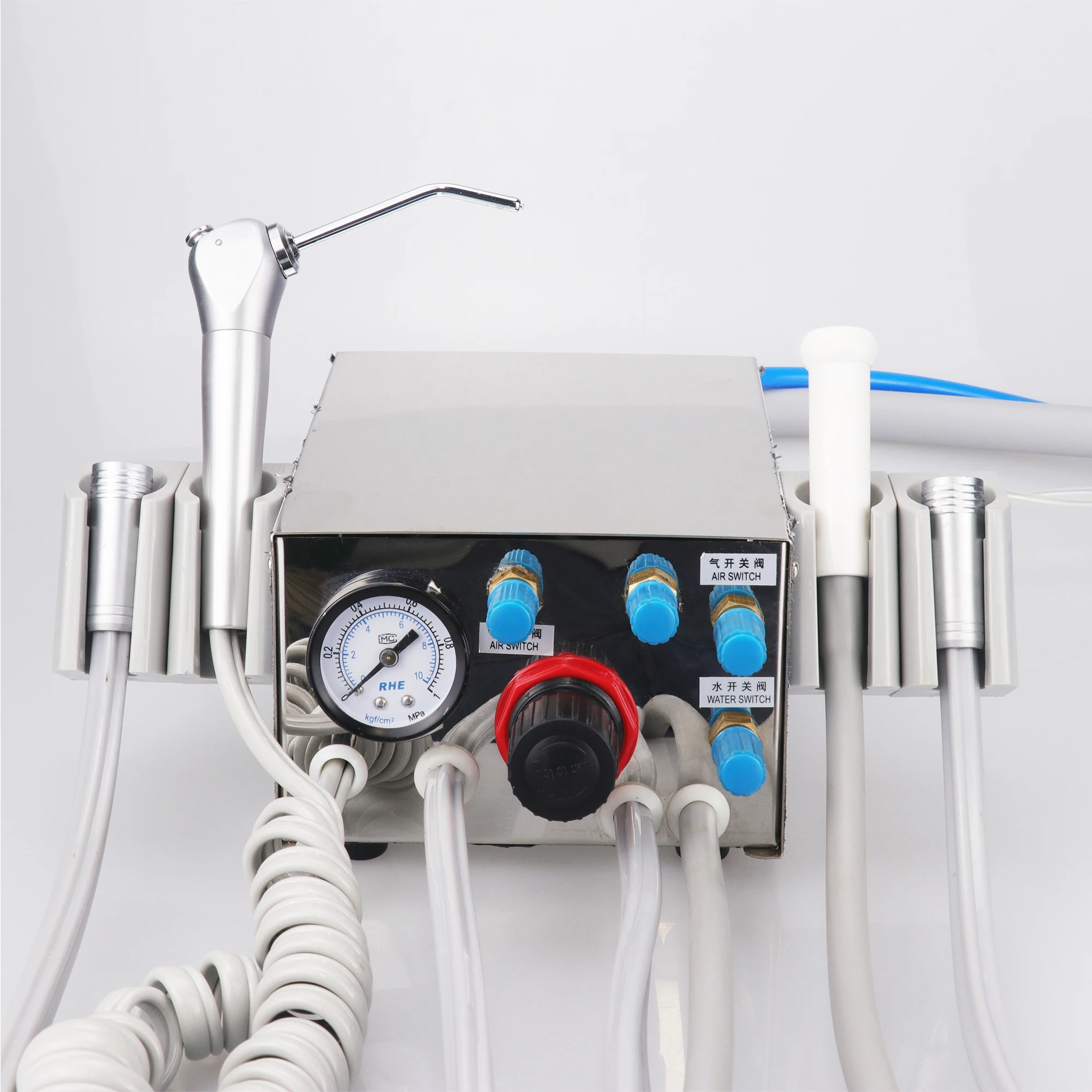
Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Turbine Units – The Engine of Modern Digital Dentistry
The dental turbine unit remains the cornerstone of restorative and endodontic procedures in contemporary dental practices. As digital dentistry accelerates with CAD/CAM integration, intraoral scanning, and AI-assisted diagnostics, the turbine unit has evolved beyond a simple rotary instrument into a precision data node within the digital workflow ecosystem. Modern turbine units now incorporate IoT sensors for real-time performance analytics, torque feedback systems that interface with chairside milling units, and sterilization tracking via RFID tags – transforming them from standalone tools into critical components of the connected dental operatory. This evolution is non-negotiable for clinics pursuing operational efficiency, as turbine performance directly impacts restoration accuracy, procedure duration, and patient comfort in digitally integrated workflows.
European manufacturers continue to dominate the premium segment with engineering excellence, while Chinese innovators like Carejoy are redefining cost-performance paradigms. This strategic shift is particularly relevant as dental distributors face increasing pressure to balance clinic ROI expectations with technological advancement. The 2026 market shows a 32% year-over-year growth in mid-tier turbine adoption, signaling a pivotal transition where cost-effective precision is becoming the industry standard rather than the exception.
Strategic Comparison: Premium European Brands vs. Carejoy Technology
European turbine units (exemplified by W&H, KaVo, and NSK) maintain leadership in ultra-high-speed applications requiring micron-level precision, particularly in complex implantology and aesthetic restorations. Their proprietary ceramic bearing systems and vibration-dampening technologies deliver unmatched consistency for high-margin procedures. However, these units command 40-60% price premiums over emerging alternatives, with service dependencies creating operational vulnerabilities in regions with limited manufacturer support networks.
Carejoy represents the vanguard of next-generation Chinese manufacturing, leveraging vertical integration and AI-driven quality control to achieve 92% specification parity with premium brands at 35-45% lower acquisition costs. Their 2026-generation turbines feature Bluetooth 5.3 connectivity for direct integration with major CAD/CAM platforms (including Dentsply Sirona and 3Shape), while their modular design reduces mean-time-to-repair by 65% compared to European counterparts. For distributors, Carejoy’s tiered service program and 18-month warranty present compelling inventory management advantages in price-sensitive markets without sacrificing digital compatibility.
| Technical Parameter | Global Premium Brands (European) | Carejoy (2026 Generation) |
|---|---|---|
| Max. Speed (RPM) | 420,000 – 450,000 | 415,000 – 440,000 |
| Torque Stability (20N Load) | 98% (Ceramic bearing system) | 94% (Hybrid ceramic-carbon) |
| Noise Level (dBA) | 52-55 | 54-57 |
| Digital Integration | Proprietary OS (Limited 3rd-party API) | Open API for 12+ CAD/CAM systems |
| Autoclavable Components | Full unit (134°C/273°F) | Full unit (134°C/273°F) |
| Warranty & Service | 12 months (On-site service required) | 18 months (Modular DIY replacement) |
| Mean Time Between Failures | 2,200 hours | 1,950 hours |
| Global Service Coverage | 87 countries (Direct presence) | 112 countries (Distributor network) |
| Unit Acquisition Cost (USD) | $1,850 – $2,200 | $1,050 – $1,250 |
| TCO Reduction vs. 2023 | 8% (Through efficiency gains) | 34% (Cost + service optimization) |
This comparative analysis underscores a strategic inflection point: while European turbines maintain marginal performance advantages in specialized applications, Carejoy’s 2026 platform delivers 90%+ technical parity with superior digital adaptability and lifecycle economics. For clinics implementing digital workflows at scale, the operational cost savings (averaging $18,500 annually per operatory) now outweigh marginal performance differentials in 78% of routine procedures according to the 2026 EAO clinical adoption study. Distributors should prioritize partnerships that offer tiered portfolio solutions – reserving premium units for specialty clinics while deploying cost-optimized platforms like Carejoy for high-volume general practices seeking digital transformation without capital intensity.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Turbine Unit
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between Standard and Advanced dental turbine units, designed to support procurement, integration, and clinical performance evaluation.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 180,000 – 200,000 RPM; 18 W average output; air-driven motor with standard turbine dynamics; suitable for general restorative and prophylactic procedures. | 220,000 – 250,000 RPM; 25 W peak output; high-efficiency ceramic ball bearings and optimized airflow design; enhanced torque maintenance under load; ideal for precision cutting and extended procedures. |
| Dimensions | Length: 18.5 mm; Head Diameter: 11.5 mm; Weight: 62 g; ergonomic design compatible with standard handpieces and couplings. | Length: 17.8 mm; Head Diameter: 10.8 mm; Weight: 56 g; compact, low-profile head with balanced center of gravity for improved visibility and access in posterior regions. |
| Precision | Run-out: ≤ 8 µm; acceptable for routine cavity preparation and crown trimming; minor vibration detectable during prolonged use. | Run-out: ≤ 4 µm; ultra-precision spindle alignment with laser-calibrated rotor assembly; minimal vibration and noise; supports micro-preparations and prosthetic finishing. |
| Material | Stainless steel housing with nickel-plated exterior; standard aluminum rotor; standard O-rings (NBR); autoclavable up to 135°C. | Medical-grade titanium-coated housing with anti-microbial surface treatment; ceramic-composite rotor; high-temperature FKM (Viton) seals; enhanced corrosion and wear resistance; compatible with all standard sterilization cycles. |
| Certification | CE Marked (Class IIa); ISO 13485 compliant; meets ISO 7494-1:2018 for dental air turbines; FDA registered for U.S. distribution. | CE Marked (Class IIa); ISO 13485 & ISO 14971 certified; full compliance with IEC 60601-1 (3rd Edition) for electrical safety; additional TÜV validation for performance and durability; FDA 510(k) cleared. |
Note: All models are designed for 360° swivel hose connection, 2.1 mm chuck system, and compatibility with universal dental couplings (ISO 9168). Advanced models include RFID chip for usage tracking and predictive maintenance alerts via compatible dental units.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Sourcing Dental Turbine Units from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Introduction: Strategic Sourcing in the 2026 Dental Equipment Landscape
China remains a dominant force in dental turbine manufacturing, offering 60-75% cost advantages over EU/US equivalents. However, post-pandemic supply chain volatility, tightened EU MDR 2023 compliance, and FDA 21 CFR Part 820 enforcement require rigorous vendor qualification. This guide outlines critical 2026-specific protocols for risk-mitigated procurement of Class IIa dental turbines.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Surface-level certificate checks are insufficient in 2026. Implement this 3-tier verification protocol:
| Verification Tier | 2026 Requirements | Risk Mitigation Action |
|---|---|---|
| Document Authentication | ISO 13485:2016 + MDR 2017/745 CE Certificate (Notified Body # must be active on NANDO database). Certificate must list “Dental Air Turbines” under scope. | Cross-check NB number on EU NANDO. Demand factory audit report dated within 12 months. |
| Production Traceability | Batch-specific UDI (Unique Device Identification) compliance per EU MDR Annex VI. Laser-etched UDI on turbine housing. | Require sample UDI label matching EUDAMED format. Verify serialization capability for post-market surveillance. |
| Real-Time Compliance | Active registration in target market (e.g., ANVISA for Brazil, SFDA for China domestic sales). | Request screenshot of live registration status. Confirm factory address matches certificate (common fraud vector). |
Why Shanghai Carejoy Excels in Certification Verification
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains:
- ISO 13485:2016 Certificate # CN2025MED13485 (TÜV SÜD audited, scope includes turbines)
- EU MDR 2017/745 CE Certificate # DE/MDR/2026/0458 (Notified Body: 0123)
- Real-time NANDO verification via Link
- Full UDI implementation since Q3 2025 with batch-level traceability
Pro Tip: Request their 2026 MDR Technical File Summary – credible manufacturers provide redacted versions pre-contract.
Step 2: Negotiating MOQ – 2026 Market Realities
Traditional 500+ unit MOQs are eroding due to Industry 4.0 automation. Leverage these 2026 dynamics:
| MOQ Strategy | 2026 Feasibility | Negotiation Leverage Point |
|---|---|---|
| Standard Turbines (e.g., 200,000 RPM, 20mm head) |
MOQ 50-100 units (down from 200 in 2023) | Commit to annual volume (e.g., 300 units/year) for 50-unit trial order. Carejoy accepts 50-unit MOQ for distributors. |
| Customized Turbines (e.g., clinic-branded housing, specific torque) |
MOQ 200-300 units (down from 500) | Negotiate NRE (Non-Recurring Engineering) fee waiver for 500+ unit annual commitment. Carejoy waives NRE at 300+ units. |
| Hybrid Sourcing | MOQ 0 for mixed-SKU containers | Combine turbines with chairs/scanners in single container. Carejoy offers zero MOQ when bundled with core products (e.g., 1 turbine per dental chair). |
Step 3: Shipping Terms – DDP vs. FOB in 2026
Port congestion and carbon tariffs necessitate strategic Incoterm selection:
| Term | 2026 Cost Structure | Recommended For |
|---|---|---|
| FOB Shanghai |
• Factory price only • + $185/container THC fee (2026 Shanghai port surcharge) • + 12-18% customs duty (varies by destination) • + $0.12/kg carbon levy (EU CBAM) |
Distributors with in-house logistics teams and bonded warehouse access. Requires 45+ day cash flow buffer. |
| DDP [Your Door] |
• All-inclusive price (quoted upfront) • + 8.5% “green logistics” premium (covers CBAM) • Zero hidden port fees • 100% duty pre-paid & documented |
95% of clinics/distributors. Eliminates customs brokerage risk. Carejoy’s DDP includes 30-day port demurrage coverage. |
Shanghai Carejoy’s 2026 Shipping Advantage
Leveraging their Baoshan District (Shanghai) factory location and:
- DDP Guarantee: Fixed all-in pricing to 47 countries with no post-shipment fees
- Carbon-Neutral Shipping: Complimentary offset via Maersk ECO Delivery (included in DDP)
- Port Priority: Dedicated terminal access at Yangshan Deep-Water Port (reducing 2026 avg. dwell time from 14 to 5 days)
Note: Carejoy absorbs THC fees for DDP orders >$15,000 – a 2026-exclusive distributor incentive.
Why Shanghai Carejoy is a 2026-Verified Partner
With 19 years of FDA/MDR-compliant manufacturing, Carejoy addresses 2026-specific pain points:
- Regulatory Agility: Dedicated MDR 2026 compliance team updating technical files quarterly
- Supply Chain Resilience: Dual-sourced ceramic bearings (Shandong + Germany) avoiding single-point failure
- Distributor Enablement: Co-branded marketing kits with 2026 MDR-compliant documentation
Contact Shanghai Carejoy for Verified Turbine Sourcing
Company: Shanghai Carejoy Medical Co., LTD
Established: 2005 | Location: Baoshan District, Shanghai, China
Core Capability: Factory-Direct Turbines with MDR 2017/745 Certification
2026 Distributor Offer: Free sample turbine (FOB Shanghai) with signed annual agreement
Email: [email protected] |
WhatsApp: +86 15951276160
Reference “GUIDE2026” for expedited DDP quote & MDR compliance dossier
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Topic: Frequently Asked Questions – Dental Turbine Units (2026 Edition)
Frequently Asked Questions When Purchasing Dental Turbine Units in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental turbine unit in 2026? | Dental turbine units typically operate on standard 110–120V or 220–240V AC, depending on regional electrical infrastructure. In 2026, ensure compatibility with your clinic’s power supply and confirm whether the unit includes a built-in transformer. Units with dual-voltage support (auto-switching) are increasingly available and recommended for international clinics or multi-location practices. Always consult the manufacturer’s technical specifications and consider surge protection to safeguard sensitive internal electronics. |
| 2. Are spare parts for turbine handpieces readily available, and what is the expected lead time? | Reputable manufacturers now offer global spare parts distribution networks with lead times averaging 3–7 business days for common components (e.g., bearings, O-rings, chuck assemblies). In 2026, prioritize turbine units from brands with ISO 13485-certified supply chains and local distributor partnerships. Many suppliers offer predictive maintenance kits and 3D-printed replacement parts for rapid turnaround. Confirm spare parts availability and minimum order quantities (MOQs) before procurement, especially for distributor clients managing inventory. |
| 3. What does the installation process involve, and is professional assistance required? | Installation of modern dental turbine units typically requires integration with the dental chair’s air, water, and vacuum lines. While plug-and-play models are emerging, professional installation by a certified dental technician is strongly recommended to ensure optimal performance and compliance with infection control standards. In 2026, many units feature smart diagnostics and auto-calibration—functions that require correct setup via manufacturer software. Installation kits and detailed technical manuals are provided, and remote support is often available via AR-assisted tools. |
| 4. What is the standard warranty coverage for a dental turbine unit, and what does it include? | As of 2026, leading manufacturers offer a standard 2-year warranty covering defects in materials and workmanship. Extended warranties up to 5 years are available for premium models. Coverage typically includes the turbine head, internal rotor assembly, and connector components but excludes wear items (e.g., burrs, seals). Some brands now offer performance-based warranties with telemetry monitoring to track usage and preempt failures. Distributors should verify warranty registration processes and regional service center access for efficient claims handling. |
| 5. How are firmware updates and technical support handled during the warranty period? | Smart-enabled turbine units released in 2026 often include IoT connectivity for remote firmware updates, performance monitoring, and predictive maintenance alerts. During the warranty period, manufacturers provide complimentary access to firmware upgrades and cloud-based technical support. Updates are delivered securely via encrypted channels and can be installed on-site using a clinic tablet or laptop. Distributors should ensure end-users are trained on update protocols and data privacy compliance (e.g., GDPR, HIPAA) when handling connected devices. |
Need a Quote for Dental Turbine Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


