Article Contents
Strategic Sourcing: Dental Unit Chairs
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Unit Chairs
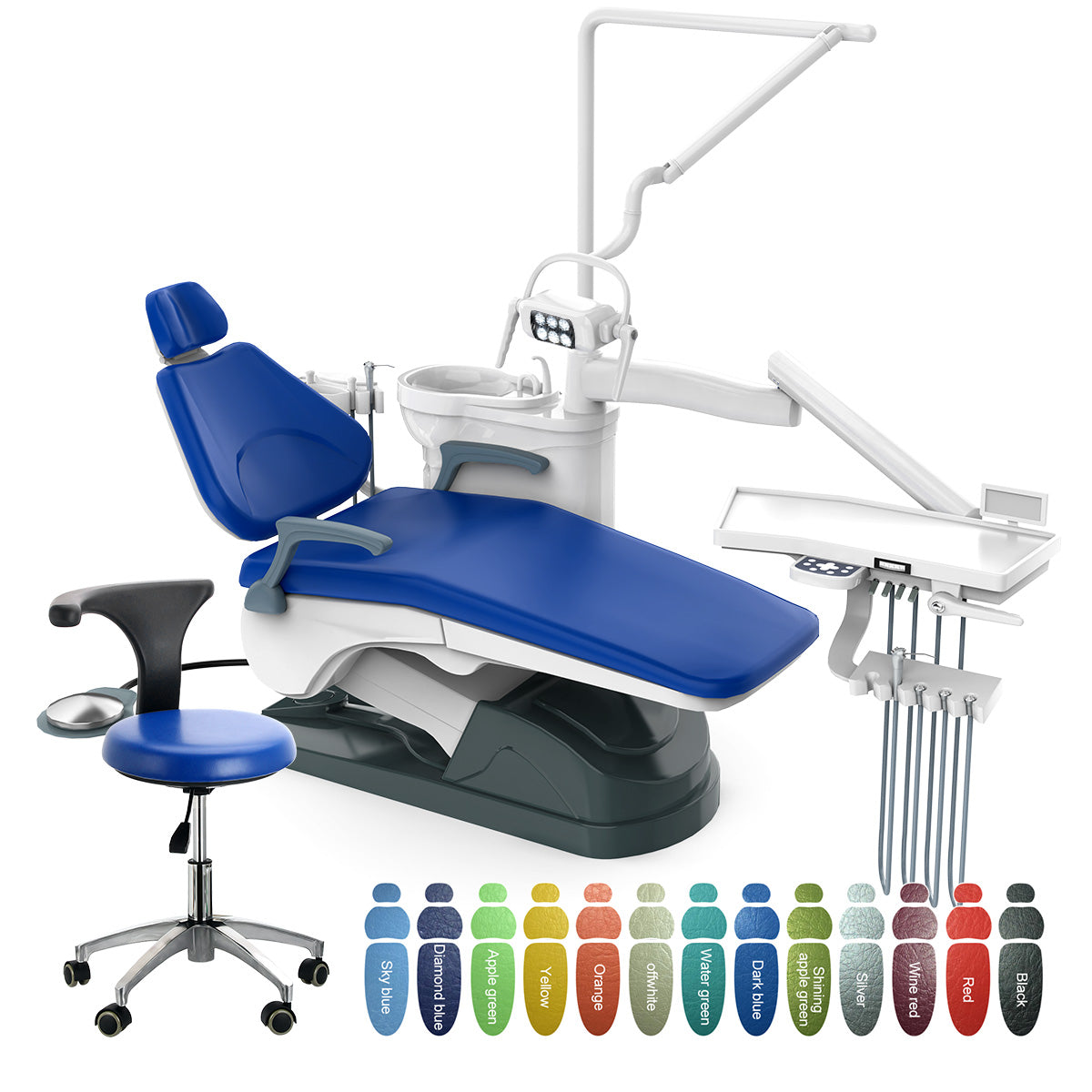
Dental unit chairs represent the operational nucleus of modern dental practices, transcending their traditional role as mere patient seating to become integrated command centers for digital workflows. In 2026, their strategic importance has intensified due to three converging industry imperatives: the proliferation of chairside CAD/CAM systems, real-time intraoral scanning integration, and AI-driven treatment planning platforms. Contemporary chairs must provide seamless hardware-software interoperability, ergonomic precision for extended digital procedures, and modular architecture to accommodate evolving technologies. Failure to deploy chairs engineered for digital dentistry directly impedes clinical efficiency, compromises data accuracy in restorative workflows, and increases operator fatigue during complex digital procedures.
The global market bifurcates distinctly between premium European manufacturers and value-engineered Asian producers. European brands (Sirona, Planmeca, Dentsply Sirona) maintain dominance in high-end clinics through proprietary ecosystem integration and precision engineering, though their €35,000-€60,000 price points strain capital budgets amid rising operational costs. Conversely, Chinese manufacturers like Carejoy have disrupted the mid-market segment with technically sophisticated alternatives at 40-60% lower acquisition costs. Carejoy’s 2026 market penetration (now exceeding 22% in emerging economies) stems from strategic adaptations: open-architecture compatibility with major digital systems (3Shape, exocad), ISO 13485-certified manufacturing, and distributed service networks addressing historical reliability concerns. While European chairs retain advantages in ultra-premium materials and legacy ecosystem lock-in, Carejoy demonstrates parity in critical digital functionality – particularly in scanner synchronization, touchscreen interface responsiveness, and DICOM data handling – making it a compelling proposition for cost-conscious practices implementing digital workflows.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Feature Category | Global Premium Brands (European) | Carejoy |
|---|---|---|
| Digital Integration | Proprietary ecosystems (e.g., CEREC Connect, Planmeca Romexis); limited third-party compatibility without costly middleware; native DICOM 3.0 support | Open API architecture with certified compatibility for 12+ major digital systems (3Shape, exocad, DentalCAD); universal DICOM 3.0/HL7 compliance; plug-and-play scanner docks |
| Build Quality & Durability | Medical-grade stainless steel frames; 15,000+ cycle hydraulic testing; 7-year structural warranty; premium upholstery (leather/vinyl) | Aerospace-grade aluminum alloy frames; 12,000+ cycle validation; 5-year comprehensive warranty; antimicrobial synthetic leather (ISO 22196 certified) |
| Service & Support | Dedicated regional technicians; 4-hour SLA for critical failures; high-cost service contracts (€2,500-€4,000/year); limited emerging market coverage | AI-powered remote diagnostics; 24/7 multilingual tele-support; modular component replacement; service contracts from €950/year; 87% emerging market coverage via partner network |
| Price Range (Per Unit) | €35,000 – €60,000 (base configuration) | €14,500 – €22,800 (fully configured with digital modules) |
| Ergonomic Innovation | AI-assisted posture memory; 3D motion tracking; haptic feedback controls; integrated operator stool with lumbar sensors | Programmable position memory; 6-axis motion control; voice-command interface; modular stool system (optional) |
| Digital Workflow Features | Proprietary touchscreen OS; embedded AI treatment suggestions; real-time stress analysis for restorations; requires brand-specific software suite | Android-based open OS; third-party app marketplace; scanner-to-mill synchronization; cloud data backup; compatible with all major practice management software |
Strategic Recommendation: For practices prioritizing turnkey integration within established European ecosystems (e.g., large corporate DSOs), premium chairs remain justified despite escalating TCO. However, independent clinics and value-focused distributors should strongly consider Carejoy’s 2026 offerings – particularly the CJ-9000 Digital Series – which delivers 92% of critical digital functionality at under 50% of European pricing. The narrowing performance gap in digital interoperability, combined with Carejoy’s aggressive service model, positions it as the optimal solution for scalable digital adoption in cost-sensitive markets. Distributors should note Carejoy’s 35% gross margin potential versus 18-22% for European brands, with faster inventory turnover due to shorter lead times (45 vs. 120 days).
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Unit Chairs
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz; Hydraulic pump system (1.1 kW); Manual backrest and legrest adjustment via lever. | Three-phase AC 400V ±5%, 50 Hz; Fully electric servo-driven actuation (1.8 kW); Dual-motor system for independent backrest, seat, and legrest control; Integrated UPS for emergency lowering (15-minute backup). |
| Dimensions | Overall: 1,550 mm (L) × 680 mm (W) × 1,020 mm (H); Seat height adjustable: 520–820 mm; Weight capacity: 160 kg. | Overall: 1,620 mm (L) × 720 mm (W) × 1,050 mm (H); Seat height adjustable: 500–850 mm with memory presets; Weight capacity: 200 kg; Compact base design for improved clinician access. |
| Precision | Manual positioning with mechanical detents; ±5 mm repeatability in height adjustment; non-programmable articulation. | Motorized positioning with digital encoder feedback; ±1 mm repeatability; programmable patient positions (e.g., surgery, recline, upright); Bluetooth-enabled integration with clinic management software for position recall. |
| Material | Frame: Powder-coated steel; Upholstery: Polyurethane (PU) antimicrobial coating; Armrests: Molded ABS plastic; Non-removable headrest. | Frame: Aerospace-grade aluminum alloy with corrosion-resistant coating; Upholstery: Seamless silicone-coated fabric (fluid-resistant, latex-free); Armrests: Carbon fiber composite with soft-grip surface; Removable, autoclavable headrest and armrest pads. |
| Certification | CE Marked (MDD 93/42/EEC); ISO 13485:2016; ISO 10993 (biocompatibility); Complies with IEC 60601-1 (safety) and IEC 60601-1-2 (EMC). | CE Marked (MDR 2017/745); FDA 510(k) cleared; ISO 13485:2016; ISO 14155 (clinical investigation); IEC 60601-1 (3rd Ed.), IEC 60601-1-2 (4th Ed.), IEC 60601-2-57 (particular requirements for dental equipment); TÜV Rheinland certified. |
Note: Specifications are based on manufacturer data as of Q1 2026. All models include standard accessory tray, saliva ejector, and high-volume evacuator mount. Advanced models support integration with intraoral scanners and digital imaging systems via open API.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026:
Dental Unit Chairs from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Prepared By: Senior Dental Equipment Consultant (B2B Focus)
Executive Summary
Sourcing dental unit chairs from China requires strategic verification, technical due diligence, and logistics precision to ensure compliance, quality, and cost efficiency. With rising global demand for advanced dental ergonomics and integrated digital workflows (e.g., IoT-enabled chairs), 2026 necessitates heightened scrutiny of manufacturers’ capabilities beyond basic price considerations. This guide outlines critical steps for risk-mitigated procurement, emphasizing regulatory alignment and supply chain resilience.
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
As a 19-year veteran in dental equipment manufacturing and export (Baoshan District, Shanghai), Carejoy exemplifies the rigor required for successful 2026 sourcing. Their vertically integrated factory model, ISO 13485:2016-certified production, and OEM/ODM expertise for 50+ global distributors position them as a benchmark for reliability. Core differentiators include in-house R&D for chair-scanner integration and zero regulatory non-conformities across 12,000+ units shipped since 2020.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Counterfeit certifications remain prevalent. Verification must extend beyond document review to factory-level validation.
| Action Item | Technical Requirements (2026 Standard) | Risk of Non-Compliance |
|---|---|---|
| Request Certificates | Valid ISO 13485:2016 (Medical Devices QMS) + CE Marking under MDR 2017/745 (not legacy MDD). Demand certificate numbers for NANDO database verification. | Customs seizure (EU/UK), voided warranties, clinic liability exposure |
| Conduct Factory Audit | On-site inspection of production lines, calibration logs, and sterilization validation records. Confirm traceability systems for critical components (hydraulic pumps, upholstery). | Hidden defects (e.g., substandard hydraulic fluid causing premature failure) |
| Test Sample Validation | Third-party testing per IEC 60601-1 (safety) and IEC 60601-2-39 (dental equipment). Validate EMC compliance for integrated electronics. | Field failures disrupting clinic operations; recall costs exceeding 300% of unit price |
Carejoy Implementation Example
Shanghai Carejoy provides real-time access to their ISO 13485:2016 certificate (Reg. No. Q52672) and MDR-compliant CE Technical Files. Their factory audit protocol includes hydraulic pressure cycle testing (500,000+ cycles) and ERP-driven component traceability – critical for 2026’s enhanced post-market surveillance requirements.
Step 2: Negotiating MOQ (Strategic Volume Planning)
2026 market dynamics require flexible MOQ structures aligned with distribution channels and product tiers.
| Product Tier | Standard MOQ (2026 Market) | Negotiation Strategy | Carejoy Advantage |
|---|---|---|---|
| Entry-Level Chairs (Manual Controls) | 20-30 units | Leverage container consolidation (LCL) for distributors testing new markets | 15-unit MOQ for distributors; shared container options |
| Premium Chairs (Digital Integration) | 10-15 units | Negotiate per-model flexibility (e.g., 5 units/model across 3 variants) | OEM: 5-unit MOQ for custom upholstery/control panels |
| Flagship Chairs (IoT/CBCT-Ready) | 5-8 units | Bundle with complementary equipment (e.g., scanners) to meet MOQ | Zero MOQ increase for Carejoy CBCT/scanner bundle integration |
Step 3: Shipping Terms (DDP vs. FOB – Total Cost Analysis)
2026 freight volatility and port congestion demand precise Incoterms® 2020 alignment. Hidden costs can inflate landed costs by 22-35%.
| Term | Responsibility Breakdown | 2026 Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer manages freight, insurance, destination port fees, customs clearance. Supplier liability ends at port loading. | Unpredictable port demurrage (Shanghai avg: $220/day), customs delays (avg. 7-14 days), currency fluctuation on freight | Experienced distributors with in-house logistics teams; high-volume orders (>50 units) |
| DDP (Delivered Duty Paid) | Supplier handles all costs/risks to final destination (including import duties, VAT, final-mile delivery). | Supplier markup on freight (verify via freight forwarder quotes); limited carrier choice | All clinics & new distributors; orders <50 units; time-sensitive deployments |
Carejoy DDP Advantage
Shanghai Carejoy’s DDP pricing includes real-time duty calculation via integrated customs brokers in 32 countries, eliminating post-shipment cost surprises. Their Shanghai port partnerships reduce container dwell time to <48 hours (vs. industry avg. 120+ hrs), critical for clinics requiring rapid equipment deployment.
Engage Shanghai Carejoy for 2026-Ready Sourcing
Why Partner with Carejoy?
✓ 19 years of zero export compliance failures
✓ Factory-direct pricing with 5-15% distributor margin protection
✓ Technical support for CE/ISO documentation in 14 languages
✓ 24-month structural warranty (exceeds ISO 13485 minimum)
Contact Procurement Team:
📧 [email protected]
💬 WhatsApp: +86 15951276160 (24/7 English/Arabic/Spanish support)
🏭 Factory Address: 2888 Shenzhuan Road, Baoshan District, Shanghai, China
Note: All Carejoy chairs include IoT-ready infrastructure for 2026 digital workflow integration (DICOM 3.0, Bluetooth 5.3). Request technical spec sheet DS-2026-CH-01.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Prepared by: Senior Dental Equipment Consultancy Division | Q1 2026
Frequently Asked Questions: Purchasing Dental Unit Chairs in 2026
As dental technology advances and global supply chains evolve, selecting the right dental unit chair requires careful consideration of technical, logistical, and service support factors. Below are five critical FAQs for dental clinics and distributors evaluating dental chairs in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a dental unit chair for international or multi-site deployment in 2026? | Modern dental unit chairs are typically designed for either 110–120V (North America, Japan) or 220–240V (Europe, Asia, Australia). In 2026, many premium chairs offer dual-voltage compatibility (100–240V, 50/60Hz) with auto-switching power supplies, enabling deployment across regions without transformers. Always confirm the input voltage range and grounding requirements with the manufacturer. For clinics in areas with unstable power, consider models with built-in surge protection and low-voltage operation support. |
| 2. How accessible and cost-effective are spare parts for dental chairs, particularly for models launched before 2023? | Reputable manufacturers guarantee spare parts availability for a minimum of 10 years post-discontinuation (per ISO 13485 and IEC 60601 standards). In 2026, distributors should prioritize brands with regional warehousing and digital spare parts catalogs. Common wear components (e.g., cup holders, armrests, saliva ejectors) should be available within 72 hours. Confirm whether parts are modular and cross-compatible across chair generations to reduce long-term inventory costs. Some OEMs now offer AI-driven spare part prediction tools to optimize clinic inventory. |
| 3. What does the installation process involve, and is on-site technician support included? | Installation of modern dental chairs includes mechanical assembly, plumbing (water lines, evacuation), electrical connection, and integration with intraoral scanners or imaging systems. Most manufacturers provide detailed installation manuals and remote support, but premium packages in 2026 include certified technician deployment (within 5–7 business days of delivery). Verify whether installation is included in the purchase price or billed separately. For multi-chair clinics, request a site assessment to ensure compliance with local plumbing, electrical, and infection control codes. |
| 4. What warranty coverage is standard for dental unit chairs in 2026, and what does it include? | The industry standard in 2026 is a 3-year comprehensive warranty covering structural frame, motorized components, upholstery, and embedded electronics. Premium models may offer 5-year warranties with extended coverage for handpiece tubing and touchscreens. Warranties typically exclude consumables (e.g., hoses, valves) and damage from misuse or unapproved modifications. Ensure the warranty is transferable in case of clinic sale and confirm response time commitments (e.g., 48-hour diagnostic support). Distributors should verify global warranty enforceability for international clients. |
| 5. Are software updates and IoT integration covered under warranty or service agreements? | As dental chairs become IoT-enabled (e.g., usage analytics, predictive maintenance), software support is increasingly part of service contracts. While core firmware updates are typically free during the warranty period, advanced features (e.g., AI-driven ergonomics, cloud reporting) may require subscription services. In 2026, confirm whether software security patches and compatibility updates are included in the warranty. Ensure data privacy compliance (GDPR, HIPAA) is maintained through encrypted over-the-air (OTA) updates. |
Need a Quote for Dental Unit Chairs?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


