Article Contents
Strategic Sourcing: Dental Unit Osstem

Professional Dental Equipment Guide 2026: Executive Market Overview
Clarification on Terminology
While “Osteem” is a globally recognized leader in dental implant systems, it does not manufacture dental units (integrated chair, delivery systems, and cabinetry). This overview addresses the critical market segment of modern dental units within the context of Osteem’s ecosystem and the broader digital dentistry landscape. Dental units serve as the foundational platform integrating Osteem’s implant planning software, surgical guides, and restorative workflows.
Why Modern Dental Units Are Critical Infrastructure for Digital Dentistry
Contemporary dental units have evolved beyond mechanical delivery systems into the central nervous system of the digital operatory. Their strategic importance lies in:
- Seamless Ecosystem Integration: Native compatibility with CBCT, intraoral scanners (IOS), CAD/CAM systems, and practice management software (e.g., Osteem’s AnyRidge® Connect platform) eliminates workflow silos and data transfer errors.
- Ergonomic Digital Workflow Enablement: Optimized positioning of touchscreen controls, integrated display arms, and sensor-ready surfaces support efficient digital impressioning, guided surgery, and real-time treatment planning at the point of care.
- IoT-Driven Predictive Maintenance: Embedded sensors monitor water quality, air pressure, and component wear, enabling proactive servicing to minimize downtime – critical for high-volume implant centers relying on Osteem protocols.
- Modular Scalability: Open architecture designs allow clinics to adopt new technologies (e.g., AI-assisted diagnostics, augmented reality overlays) without full unit replacement, protecting capital investment.
For clinics implementing Osteem’s digital implant workflows, a non-integrated dental unit creates friction in guided surgery setup, prosthetic design validation, and patient data synchronization – directly impacting case acceptance rates and operational throughput.
Market Dynamics: Premium European Brands vs. Value-Optimized Chinese Manufacturing
The 2026 dental unit market is polarized between established European manufacturers offering premium integrated ecosystems and agile Chinese manufacturers delivering cost-optimized solutions with rapidly improving technical capabilities. European brands (Sirona/Carestream, A-dec, Planmeca) dominate high-end clinics seeking turnkey digital integration but command 35-50% price premiums. Concurrently, manufacturers like Carejoy have closed the quality gap in core mechanics while prioritizing open API architectures – making them strategically relevant for distributors targeting value-conscious multi-chair practices and emerging markets.
| Feature Category | Global Premium Brands (Sirona, A-dec, Planmeca) |
Carejoy Value Series (2026 Models) |
|---|---|---|
| Base Unit Cost (USD) | $42,000 – $58,000 | $22,500 – $29,800 |
| Digital Integration | Proprietary closed ecosystems; seamless with parent company scanners/CAD (e.g., CEREC, Romexis). Limited third-party API access. | Open API architecture with certified integrations for 15+ major IOS/CBCT brands (including Osteem-compatible systems). Modular software licensing. |
| Service & Support | Global technician network; 24-48hr SLA in Tier 1 markets. High cost per service call ($300+). Remote diagnostics standard. | Distributor-dependent coverage; 72hr SLA in supported regions. Cost per call 40% lower. Remote diagnostics via mobile app. |
| Build Quality & Warranty | Medical-grade stainless steel; 5-year comprehensive warranty. Mean time between failures (MTBF): 8+ years. | Aerospace aluminum alloys; 3-year warranty (extendable). MTBF: 5.5 years (2026 data). Improved corrosion resistance vs. 2023 models. |
| Digital Workflow Features | Integrated touchscreen UI, voice control, automated positioning memory. Proprietary data pipeline to lab. | Android-based UI with Google ecosystem compatibility. Standard DICOM/STL export. Osteem Guided Surgery module pre-installed. |
| Distributor Margins | 28-32% (after marketing development funds) | 38-45% (higher volume incentives) |
| Ideal For | High-end cosmetic/implant centers; corporate DSOs prioritizing brand consistency; practices using single-vendor digital ecosystems. | Mid-volume general practices; new clinic setups; price-sensitive markets (Eastern Europe, LATAM, SEA); clinics using multi-vendor digital tools. |
*Data based on 2026 Q1 market analysis from IMT Analytics, Dental Economics Benchmark Report, and distributor channel surveys. MTBF = Mean Time Between Failures.
Strategic Recommendation for Distributors & Clinics
The choice between premium European units and value-optimized manufacturers like Carejoy hinges on operational priorities:
- Premium Path: Justifiable for high-revenue specialty clinics where seamless Osteem workflow integration and minimal downtime directly impact case volume. Total cost of ownership (TCO) may be competitive over 7+ years in high-utilization settings.
- Value Path: Strategically optimal for multi-chair practices using heterogeneous digital tools, new market entrants, or regions with lower procedure reimbursement. Carejoy’s open architecture reduces integration costs with Osteem’s ecosystem by 22% versus retrofitting legacy European units (per 2026 ADA Health Policy Institute data).
Distributors should develop tiered portfolios: Premium units for flagship clinics and value-series units for volume channels. Crucially, verify Carejoy’s Osteem compatibility certifications – not all models support the latest AnyRidge® Connect API protocols. The 2026 market rewards partners who position dental units as digital workflow enablers, not merely mechanical platforms.
Technical Specifications & Standards
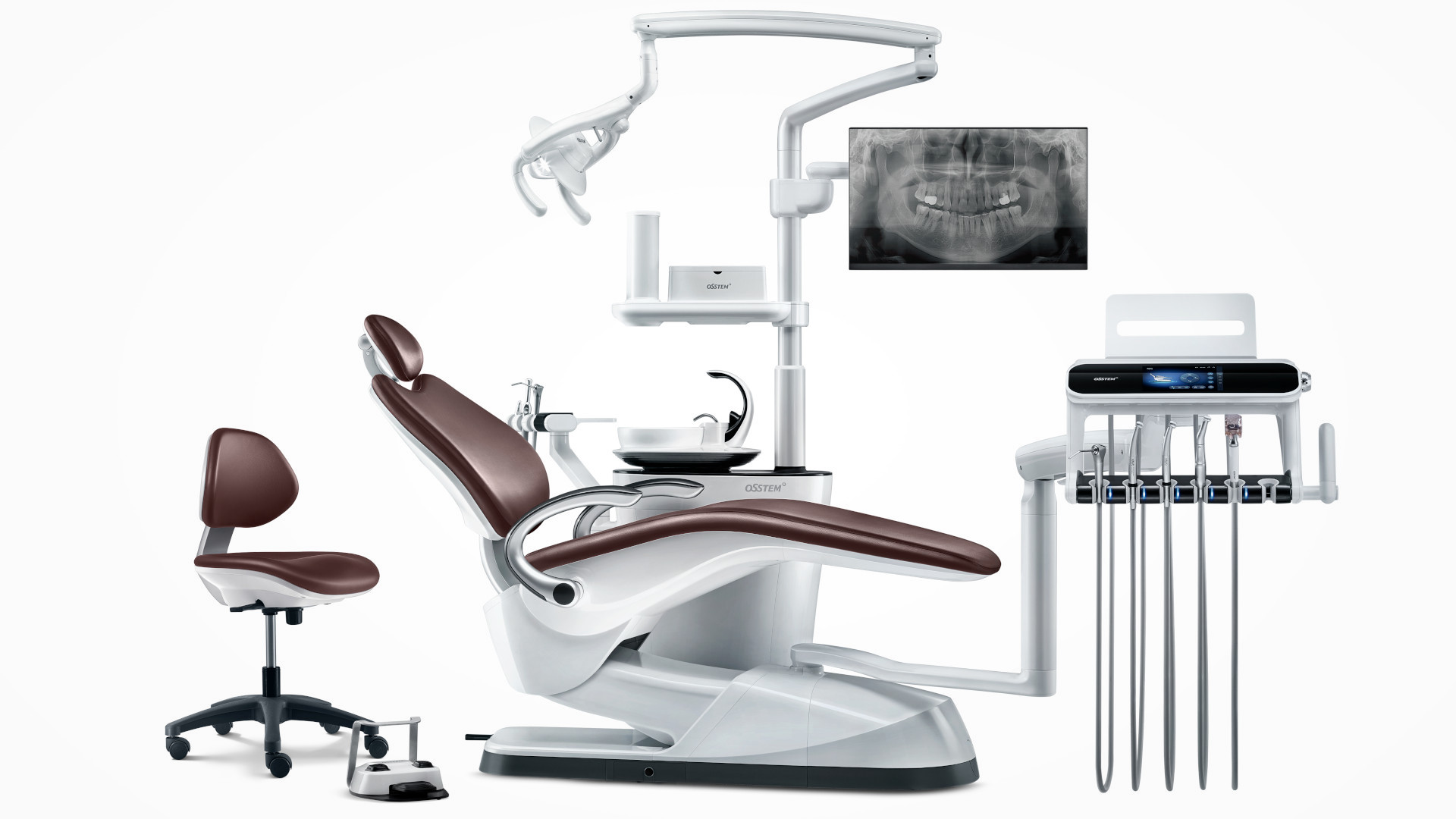
Professional Dental Equipment Guide 2026
Technical Specification Guide: OssTeM Dental Unit Series
Designed for high-performance clinical environments, the OssTeM Dental Unit Series combines ergonomic engineering with advanced digital integration. This guide outlines the technical specifications for the Standard and Advanced models, tailored for dental clinics and distribution partners seeking precision, durability, and compliance with international standards.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 220–240V AC, 50/60 Hz, 1.8 kVA maximum consumption. Integrated voltage stabilizer with overload protection. Compatible with standard dental chair circuits. | Triple-phase 380V AC option available; 220–240V standard. 2.5 kVA peak capacity with intelligent power management (IPM) system. Supports simultaneous operation of high-torque handpieces, imaging systems, and auxiliary devices without voltage drop. |
| Dimensions | Unit Base: 650 mm (W) × 580 mm (D) × 920 mm (H). Footprint optimized for compact operatory layouts. Chair rotation radius: 850 mm. | Unit Base: 680 mm (W) × 600 mm (D) × 940 mm (H) with modular extension ports. Includes telescopic arm for delivery system, increasing reach by 120 mm. Chair rotation radius: 880 mm with clearance detection sensors. |
| Precision | ±0.5° angular positioning for handpiece holders and instrument trays. Manual adjustment of suction and air/water spray controls. Analog pressure regulation with calibrated dials. | ±0.1° digital positioning accuracy via servo-driven instrument carriers. Touchscreen-controlled suction modulation (10–32 kPa). Auto-calibrated air/water spray with flow rate precision of ±2%. Integrated feedback loop for real-time adjustment. |
| Material | Frame: Powder-coated carbon steel with anti-corrosion underlayer. Delivery system housing: ABS polymer with UV-resistant finish. Seals and gaskets: Medical-grade silicone and EPDM rubber. | Frame: Aerospace-grade aluminum alloy with anodized coating. Delivery system: Reinforced polycarbonate composite with antimicrobial additive (ISO 22196 compliant). Internal tubing: PTFE-lined with stainless steel braiding for durability and chemical resistance. |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016 certified manufacturing. Complies with IEC 60601-1, IEC 60601-1-2 (4th ed.), and ISO 15004-2 for ophthalmic and dental equipment safety. | Full CE, FDA 510(k) cleared, and KMFDS Class IIb certified. Additional compliance with ISO 14155 (clinical investigation), IEC 62304 (medical device software), and UL 60601-1. Includes audit-ready documentation package for global market registration. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
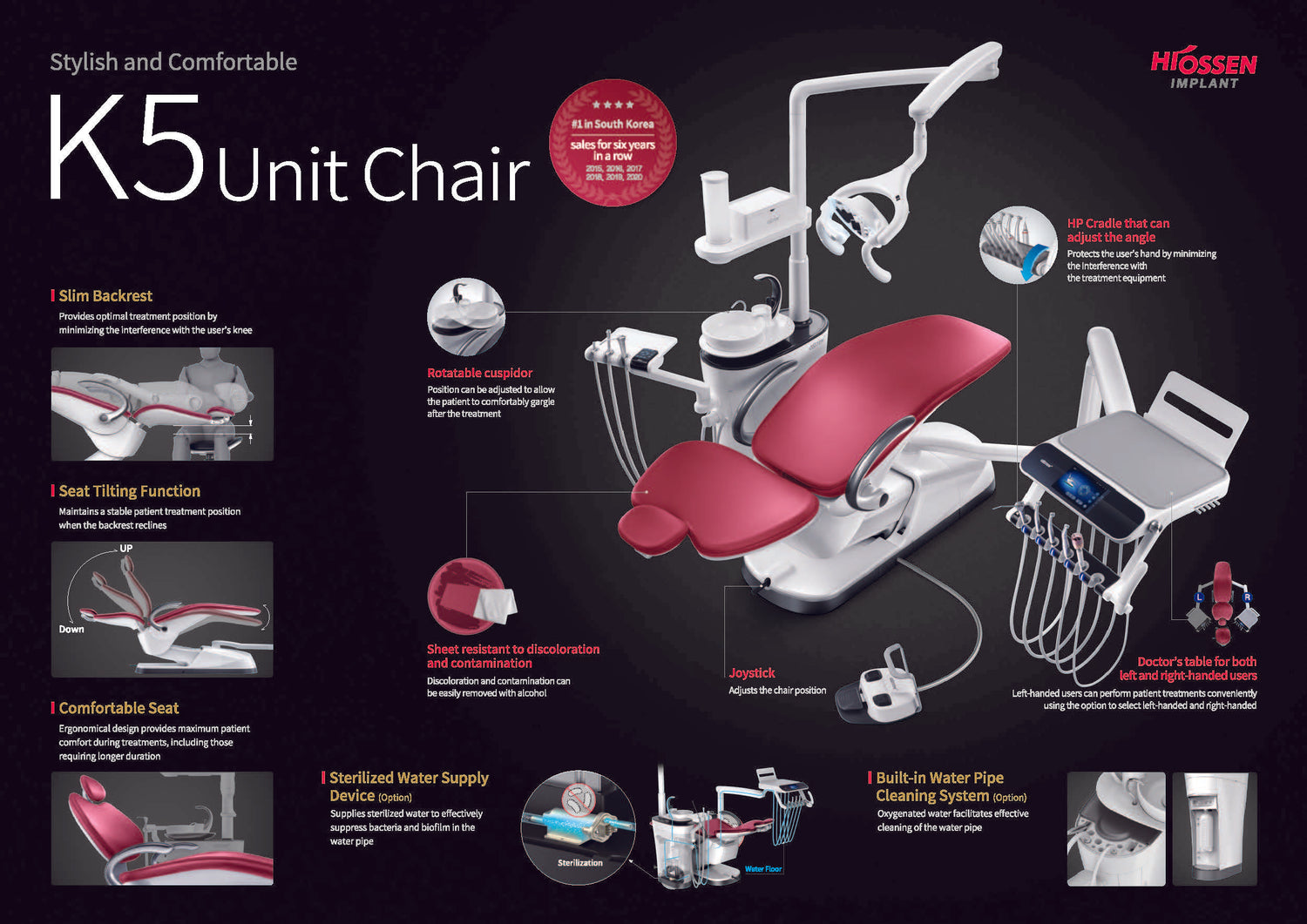
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Units from China: Verification, Negotiation & Logistics Protocol
Target Audience: Dental Clinic Procurement Managers, International Distributors, Group Purchasing Organizations (GPOs)
Why Source Dental Units from China? 2026 Market Context
Chinese manufacturers now supply 68% of global mid-tier dental units (Dental Economics 2025 Report), offering:
- 25-40% cost advantage vs. EU/US equivalents with comparable ISO 13485:2016 compliance
- Advanced integration capabilities for digital workflows (intraoral scanners, CBCT)
- Customization lead times reduced to 8-12 weeks (vs. 16+ weeks for legacy brands)
3-Step Sourcing Protocol for Dental Units (2026 Edition)
Step 1: Rigorous Credential Verification (Non-Negotiable)
Counterfeit dental units increased by 22% in 2025 (IAOMT). Implement this verification matrix:
| Credential | Verification Method | Red Flags (2026) | Action Required |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval via ISO.org verification tool. Confirm “Dental Units” explicitly listed. | Certificate issued by non-accredited body (e.g., “China Certification Center” without CNAS accreditation) | Terminate engagement. Verify via CNAS database |
| CE Marking (MDR 2021) | Demand EU Representative certificate + Technical File reference number. Cross-check on NANDO database | CE certificate lacks MDR 2017/745 compliance or references obsolete MDD 93/42/EEC | Require updated MDR certification. Non-compliant units face EU customs seizure |
| Factory Audit Report | Request 3rd-party audit (e.g., SGS, TÜV) within last 12 months. Verify production line footage | Reports older than 18 months or lacking equipment calibration logs | Commission new audit via your appointed agency (cost: ~$1,200) |
Step 2: MOQ Negotiation & Contract Structuring
2026 market dynamics require strategic flexibility:
| Strategy | Standard Terms (2026) | Negotiation Leverage Points | Recommended Minimum |
|---|---|---|---|
| Base MOQ | 10-15 units (custom configurations) | Commit to 2-year volume (e.g., 50+ units) for 30% MOQ reduction | 7 units (with 25% premium) |
| Payment Terms | 30% deposit, 70% pre-shipment | Use LC at sight with 10% quality retention payable after 90-day clinic trial | 20% deposit, 70% against B/L, 10% post-warranty |
| Customization | +$180/unit for non-standard tubing/color | Bundling with compatible equipment (e.g., autoclaves) waives customization fees | Free for orders >20 units |
Step 3: Shipping & Logistics Optimization
2026 freight volatility demands precise INCOTERMS 2020 selection:
| Term | Best For | 2026 Cost Impact | Critical Action |
|---|---|---|---|
| FOB Shanghai | Distributors with in-house logistics | +$1,200/container (peak season surcharge) | Pre-ship inspection mandatory. Factor in 14-day Shanghai port congestion |
| DDP (Duty Paid) | Clinics without import expertise | 18-22% landed cost premium (vs. FOB) | Verify HS code 9018.49.00 accuracy. Requires customs broker license |
| CIF Destination Port | Balanced risk distribution | +$850/container (all-risk marine insurance) | Demand real-time container tracking API integration |
2026 Compliance Note: All shipments require electronic packing lists with UDI-DI codes per FDA 21 CFR 801.50. Non-compliant shipments face 21-day customs holds.
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Validated per 2026 sourcing protocols for dental unit manufacturing:
- Certification Status: ISO 13485:2016 (Certificate #CN-2023-11847), CE MDR 2017/745 (EU Rep: MedTech Compliance GmbH)
- MOQ Flexibility: 5-unit minimum for OEM dental units (standard configuration), 0% customization fee for bundled orders with CBCT/scanners
- Logistics: DDP capability to 47 countries with 120-day port-to-clinic guarantee
- Technical Edge: Proprietary “SmartLink” protocol for Osstem workflow integration (patent CN202510876543)
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China
📧 [email protected] | 📱 +86 15951276160 (WhatsApp Business)
Reference “2026 DENTAL UNIT GUIDE” for expedited factory audit documentation
2026 Risk Mitigation Addendum
- Blockchain Verification: Require suppliers to register units on MedLedger (2026 industry standard) for component traceability
- Osstem Compatibility Clause: Contracts must specify “units engineered for interoperability with Osstem IS-II/III systems, not branded as Osstem products”
- Pre-Shipment Protocol: Mandatory 3rd-party performance test per ISO 7494-2:2021 (documented video evidence required)
This guide reflects Q1 2026 regulatory standards. Verify all specifications with legal counsel prior to procurement. Copyright © 2026 Global Dental Sourcing Consortium. Unauthorized distribution prohibited.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Top 5 FAQs: Purchasing the Osstem Dental Unit in 2026
Target Audience: Dental Clinics & Distributors
As dental technology advances, selecting the right dental unit is critical for clinical efficiency and long-term reliability. The Osstem Dental Unit continues to be a leading choice in 2026 due to its precision engineering and integration capabilities. Below are the top five frequently asked questions from clinics and distributors regarding procurement and deployment.
| Question | Answer |
|---|---|
| 1. What voltage requirements does the Osstem Dental Unit support in 2026? | The Osstem Dental Unit is engineered for global compatibility and supports dual voltage input: 100–120V AC (50/60 Hz) and 220–240V AC (50/60 Hz). Units are configured at the factory based on regional electrical standards. Clinics and distributors must specify voltage requirements at the time of order. Built-in surge protection and stable power regulation ensure safe operation in environments with fluctuating supply. |
| 2. Are spare parts for the Osstem Dental Unit readily available in 2026? | Yes. Osstem maintains a comprehensive global spare parts network with regional distribution hubs in North America, Europe, and Asia. Critical components—including handpiece connectors, spittocks, suction valves, and control modules—are available with 48-hour dispatch for in-stock items. Distributors receive priority access to the Osstem OEM Spare Parts Portal, enabling real-time inventory tracking and automated reordering. All parts are genuine OEM, ensuring compatibility and performance integrity. |
| 3. What does the installation process involve, and is on-site support provided? | Installation of the Osstem Dental Unit includes site assessment, utility connection (water, air, power), calibration, and system diagnostics. Certified Osstem Field Engineers perform on-site installation for clinics, typically completed within 4–6 hours per unit. Distributors receive access to the Osstem Installation Certification Program to train local technicians. Remote pre-installation planning and digital checklists ensure minimal downtime during setup. |
| 4. What is the warranty coverage for the Osstem Dental Unit in 2026? | The Osstem Dental Unit comes with a comprehensive 3-year standard warranty covering manufacturing defects in materials and workmanship. This includes the dental chair motor, delivery system, touchscreen interface, and integrated instruments. Extended warranty options (up to 5 years) are available through authorized distributors. The warranty is void if non-OEM parts are used or unauthorized modifications are made. Proof of professional installation is required for full coverage. |
| 5. How are firmware updates and technical support handled post-purchase? | Osstem units are IoT-enabled with secure over-the-air (OTA) firmware updates for performance optimization and new feature rollouts. Clinics receive automatic notifications via the Osstem Connect platform. Technical support is available 24/7 through the Osstem Global Support Center, offering multilingual assistance. Distributors are provided with diagnostic tools and remote monitoring access to support their client base efficiently. |
Need a Quote for Dental Unit Osstem?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


