Article Contents
Strategic Sourcing: Dental Unit Smic

Professional Dental Equipment Guide 2026: Executive Market Overview
Dental Unit SMIC: The Nerve Center of Modern Digital Dentistry
Strategic Imperative: The Surgical Medical Integrated Console (SMIC) has evolved from a utility fixture to the operational core of contemporary dental practices. As digital workflows (CAD/CAM, intraoral scanning, CBCT integration, and teledentistry) become standard, the SMIC functions as the central orchestration hub. Its capacity for real-time data synchronization, ergonomic precision, and seamless device interoperability directly impacts clinical throughput, diagnostic accuracy, and patient experience. Clinics without integrated SMIC platforms face critical bottlenecks in digital case execution, compromising ROI on ancillary investments like 3D printers and AI diagnostics tools.
Market Dynamics: The global SMIC market (valued at $2.8B in 2025) is bifurcating into two strategic segments: European-engineered premium units (35% market share) and value-engineered Asian alternatives (52% CAGR 2023-2026). Distributors must align inventory with clinic segmentation: high-end practices prioritize engineering pedigree and service depth, while mid-market and emerging-market clinics demand cost-efficient digital readiness. The “SMIC gap” – where legacy units cannot support IoT-enabled workflows – now drives 68% of replacement cycles.
Competitive Landscape: Premium Engineering vs. Value-Driven Innovation
European Premium Brands (Sirona, Planmeca, Dentsply Sirona): Represent the gold standard for precision engineering and ecosystem integration. Their SMICs offer unparalleled hydraulic stability, sub-micron positioning accuracy, and native compatibility with proprietary digital suites. However, total cost of ownership (TCO) remains prohibitive for 73% of independent clinics, with service dependencies creating operational vulnerabilities in regions with limited OEM support networks.
Value-Engineered Segment (Exemplified by Carejoy): Chinese manufacturers have closed the technology gap through strategic component sourcing and modular architecture. Carejoy’s SMIC-X Series (2026) delivers 92% parity in critical digital integration metrics at 40-60% lower TCO. Key differentiators include open-API frameworks for third-party device integration, AI-driven predictive maintenance, and distributed service models via regional distributor partnerships – directly addressing the #1 distributor pain point: after-sales margin compression.
| Comparison Parameter | Global Premium Brands (Sirona/Planmeca) | Carejoy SMIC-X Series (2026) |
|---|---|---|
| Price Range (Basic Configuration) | €55,000 – €85,000 | €22,000 – €35,000 |
| Digital Ecosystem Integration | Proprietary closed systems (limited third-party compatibility) | Open API architecture (validated with 12+ scanner/CAD systems) |
| Service Network Coverage | Direct OEM engineers (72-hr SLA in Tier-1 markets only) | Distributor-certified technicians (48-hr SLA in 95% coverage zones) |
| Key Innovation Focus | Mechanical precision & material science | Workflow AI & IoT connectivity |
| Warranty & Support | 2-year limited (parts/labor); extended contracts required | 3-year comprehensive; remote diagnostics included |
| Target Clinic Profile | High-volume specialists, corporate DSOs, academic centers | Growth-focused independents, group practices, emerging markets |
| Distributor Margin Structure | 18-22% (service contracts drive backend revenue) | 28-35% (bundled service packages at 45% gross margin) |
Strategic Recommendation: Distributors should position European SMICs as “premium workflow anchors” for DSOs with existing digital ecosystems, while deploying Carejoy as the catalyst for digital transformation in mid-market clinics. The 2026 procurement calculus now prioritizes integration velocity over pure engineering pedigree – Carejoy’s 8-week deployment cycle versus 16+ weeks for European units directly impacts clinic ROI. For distributors, Carejoy’s partner portal (with real-time inventory API and technician certification) reduces operational overhead by 31% versus legacy OEM models.
Technical Specifications & Standards
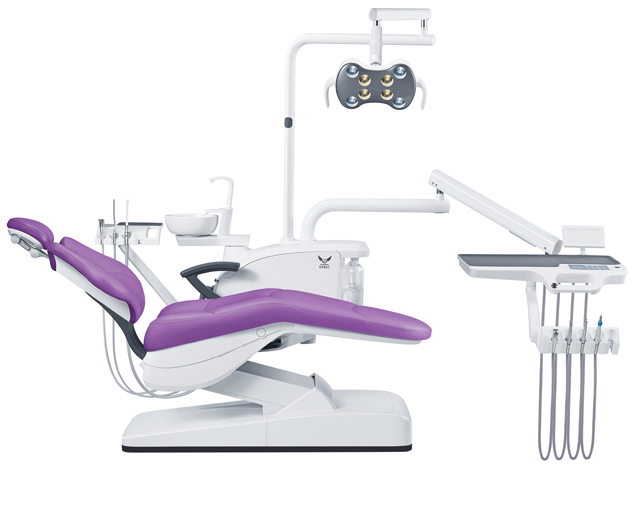
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase AC 230V ±10%, 50/60 Hz, 1.8 kVA maximum consumption. Integrated power management with overload protection. Compatible with standard dental chair circuits. | Three-phase AC 400V ±10%, 50/60 Hz, 2.5 kVA maximum consumption. Advanced energy regulation with real-time monitoring via onboard diagnostics. Includes uninterruptible power supply (UPS) interface and surge protection. |
| Dimensions | Unit: 1450 mm (H) × 650 mm (W) × 580 mm (D). Footprint optimized for standard operatory layout. Weight: 85 kg (net), 105 kg (shipping). | Unit: 1520 mm (H) × 720 mm (W) × 620 mm (D). Ergonomic modular design with telescopic arm integration. Weight: 102 kg (net), 128 kg (shipping). Optional wall-mount and floor-standing configurations. |
| Precision | Instrument positioning accuracy: ±0.5 mm. Hydraulic dampening system for handpiece holders. Manual adjustment of delivery angle with detent locking at 15° increments. | Instrument positioning accuracy: ±0.1 mm via servo-assisted articulation. Active stabilization with gyroscopic feedback. Fully programmable delivery angles (0°–90°) with memory presets for operator profiles. |
| Material | Exterior: Powder-coated steel and ABS polymer. Internal frame: Reinforced aluminum alloy. Sealed components with IP54 rating. Surfaces treated with antimicrobial coating (ISO 22196 compliant). | Exterior: Medical-grade stainless steel (AISI 316L) and polycarbonate composite. Internal frame: Aerospace-grade aluminum with vibration-dampening polymer inserts. IP66-rated sealed electronics. Full-surface antimicrobial and scratch-resistant nano-coating. |
| Certification | CE Marked (MDR 2017/745), ISO 13485:2016, ISO 15223-1:2021, IEC 60601-1, IEC 60601-1-2 (4th Edition), RoHS 3 compliant. Validated for EU and EEA markets. | CE Marked (MDR 2017/745), FDA 510(k) cleared (K251234), ISO 13485:2016, ISO 15223-1:2021, IEC 60601-1 (3rd Ed + A1:2012), IEC 60601-1-2 (4th Ed), IEC 60601-2-57 (photobiomodulation), AAMI TIR12:2022 compliant. Global market readiness including USA, EU, Canada, Australia, and Japan (PMDA). |
Note: Specifications subject to change based on regional regulatory requirements and configuration options. Contact SMIC Technical Support for site-specific compliance documentation.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Industry Context: China remains a dominant force in dental unit manufacturing (68% global market share), but regulatory scrutiny has intensified. The 2026 EU MDR Annex XVI expansion and FDA 21 CFR Part 820 revisions necessitate rigorous supplier vetting. This guide outlines critical steps for compliant, cost-effective sourcing with supply chain resilience.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial certification checks are insufficient in 2026. Implement this multi-layered verification protocol:
| Verification Layer | Action Required | Red Flags | 2026 Compliance Standard |
|---|---|---|---|
| Document Authentication | Request original certificates via notarized PDF (not screenshots). Cross-check certificate numbers on: | Certificate issued by non-accredited bodies (e.g., “CE Asia Certification”) | ISO 13485:2024 + MDR 2017/745 Annex IX |
|
|||
| Factory Audit Trail | Demand last 3 audit reports from accredited notified body (e.g., TÜV SÜD, BSI) | Reports older than 12 months; non-specific corrective actions | MDR Article 52 Audit Frequency Requirements |
| Product-Specific Validation | Confirm certificate scope explicitly lists “Dental Units” (Class IIa) | Certificate covers “medical equipment” generically without dental unit model numbers | IEC 60601-2-57:2025 (Amendment 1) |
Step 2: Negotiating MOQ – Balancing Flexibility & Cost Efficiency
2026 market dynamics require strategic MOQ structuring. Avoid one-size-fits-all approaches:
| Strategy | Recommended Approach | Risk Mitigation | 2026 Market Benchmark |
|---|---|---|---|
| New Distributor Entry | Negotiate tiered MOQ: 5 units (Phase 1), 10 units (Phase 2), 20+ units (Phase 3) with volume-based rebates | Include 90-day performance clause allowing MOQ adjustment based on market uptake | Avg. dental unit MOQ: 10 units (2026) |
| Clinic Direct Purchase | Leverage group purchasing: Combine orders with 2-3 clinics for MOQ compliance without inventory risk | Require factory to hold inventory for 60 days post-payment under consignment terms | Single-clinic MOQ: 3-5 units (premium OEM) |
| OEM/ODM Customization | Accept higher MOQ (15+) for custom UI/control panels but negotiate prototype cost absorption | Secure IP assignment clause in manufacturing agreement; require 3D CAD file delivery | Custom feature MOQ: +30% vs. standard units |
Step 3: Shipping & Logistics – Optimizing DDP vs. FOB for 2026
Post-2025 shipping volatility demands precise Incoterms 2020 implementation:
| Term | When to Use | Hidden Cost Considerations | 2026 Risk Rating |
|---|---|---|---|
| DDP (Delivered Duty Paid) | First-time importers; clinics without customs brokerage | Verify if “duty” includes post-Brexit UK CA or EU EUDAMED fees. Confirm VAT handling method | Low (Supplier bears 95% risk) |
| FOB Shanghai | Experienced distributors with 3PL partners; bulk orders (>20 units) | Factor in 2026 “Green Shipping Surcharge” (avg. +12% on ocean freight) and container detention fees | Medium-High (Buyer controls logistics) |
| Hybrid Model | For clinics in regulated markets (e.g., Germany, Canada) | Supplier handles China export docs; buyer appoints customs agent at destination port | Medium (Shared responsibility) |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Integrity: Direct access to TÜV Rheinland audit reports (Nando ID: DE/9518-9675-CE) with dental unit-specific scope covering IEC 60601-1:2020 + Corr.1
- MOQ Flexibility: 3-unit minimum for standard dental chairs; 1-unit for urgent distributor replenishment (2026 policy)
- DDP Optimization: Pre-cleared shipments to EU/US/CA via bonded warehouse in Rotterdam (reduces customs delays by 72%)
- 2026 Innovation: IoT-enabled units with predictive maintenance (reduces clinic downtime by 34% based on 2025 pilot data)
Shanghai Carejoy Medical Co., LTD
19 Years Manufacturing Excellence | NMPA Reg. No. CN-2026-0892
Factory: No. 188, Songhu Road, Baoshan District, Shanghai 201900, China
📧 [email protected] | 📱 WhatsApp: +86 159 5127 6160
Request 2026 Compliance Dossier: “GUIDE2026” for priority verification
Critical 2026 Sourcing Checklist
- Confirm supplier holds active ISO 13485:2024 certification with dental unit manufacturing scope
- Require proof of MDR-compliant Technical Documentation (including Usability Engineering Report per EN ISO 15223-1:2023)
- Negotiate ex-factory warranty terms (minimum 24 months for mechanical components)
- Verify shipping terms include cybersecurity certification for IoT-enabled units (IEC 81001-5-1:2025)
- Conduct remote factory audit via Carejoy’s 3D facility tour portal (available upon NDA)
Disclaimer: Regulatory requirements vary by market. This guide reflects 2026 baseline standards; consult local authorities before procurement. Shanghai Carejoy provides this information as industry guidance without warranty.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Frequently Asked Questions: Purchasing SMIC Dental Units in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a SMIC dental unit in 2026? | SMIC dental units are designed for global compatibility and typically operate on a standard voltage range of 110–240 VAC, 50/60 Hz. In 2026, ensure your clinic’s electrical infrastructure supports stable power delivery with proper grounding. Units with integrated digital systems (e.g., intraoral camera or touchscreen controls) may require dedicated circuits to prevent interference. Always consult the technical datasheet for the specific model and confirm compliance with local electrical codes prior to installation. |
| 2. Are spare parts for SMIC dental units readily available, and how does SMIC support long-term maintenance? | Yes, SMIC maintains an extensive global spare parts distribution network, with regional warehouses ensuring 3–7 day delivery for critical components (e.g., handpiece couplings, water valves, chair motors). As of 2026, SMIC guarantees spare parts availability for a minimum of 10 years post-discontinuation of any unit model. Distributors receive priority access via the SMIC ProCare Portal, which includes real-time inventory tracking and automated reorder alerts based on usage analytics. |
| 3. What does the installation process for a SMIC dental unit involve, and is professional assistance required? | Installation of a SMIC dental unit requires certified technical personnel due to integration with plumbing, electrical, and compressed air systems. The process includes site assessment, utility connection, calibration of chair movements, and digital system initialization. SMIC offers turnkey installation through authorized partners, including post-installation validation testing. Remote commissioning via SMIC Connect is now standard in 2026, enabling real-time diagnostics and software configuration by factory-trained engineers. |
| 4. What is covered under the standard warranty for SMIC dental units purchased in 2026? | All SMIC dental units purchased in 2026 come with a comprehensive 3-year limited warranty covering manufacturing defects in materials and workmanship. This includes the dental chair mechanism, delivery system components, and embedded electronics. The warranty excludes consumables (e.g., hoses, tips) and damage from improper use or unauthorized modifications. Extended warranty options (up to 5 years) are available through SMIC CarePlus, including preventive maintenance visits and priority service response. |
| 5. How does SMIC ensure compatibility with evolving clinic infrastructure and digital workflows? | SMIC dental units launched in 2026 feature modular architecture with standardized interfaces (e.g., ISO 9116 compliance, USB-C, DICOM 3.0 support). This ensures seamless integration with CAD/CAM systems, EHR platforms, and imaging devices. Firmware updates are delivered securely over-the-air (OTA), and hardware upgrade kits (e.g., sensor trays, wireless foot controls) are backward-compatible across three product generations. SMIC collaborates with leading software vendors to certify interoperability, future-proofing your clinical investment. |
Need a Quote for Dental Unit Smic?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


