Article Contents
Strategic Sourcing: Dental Units For Sale
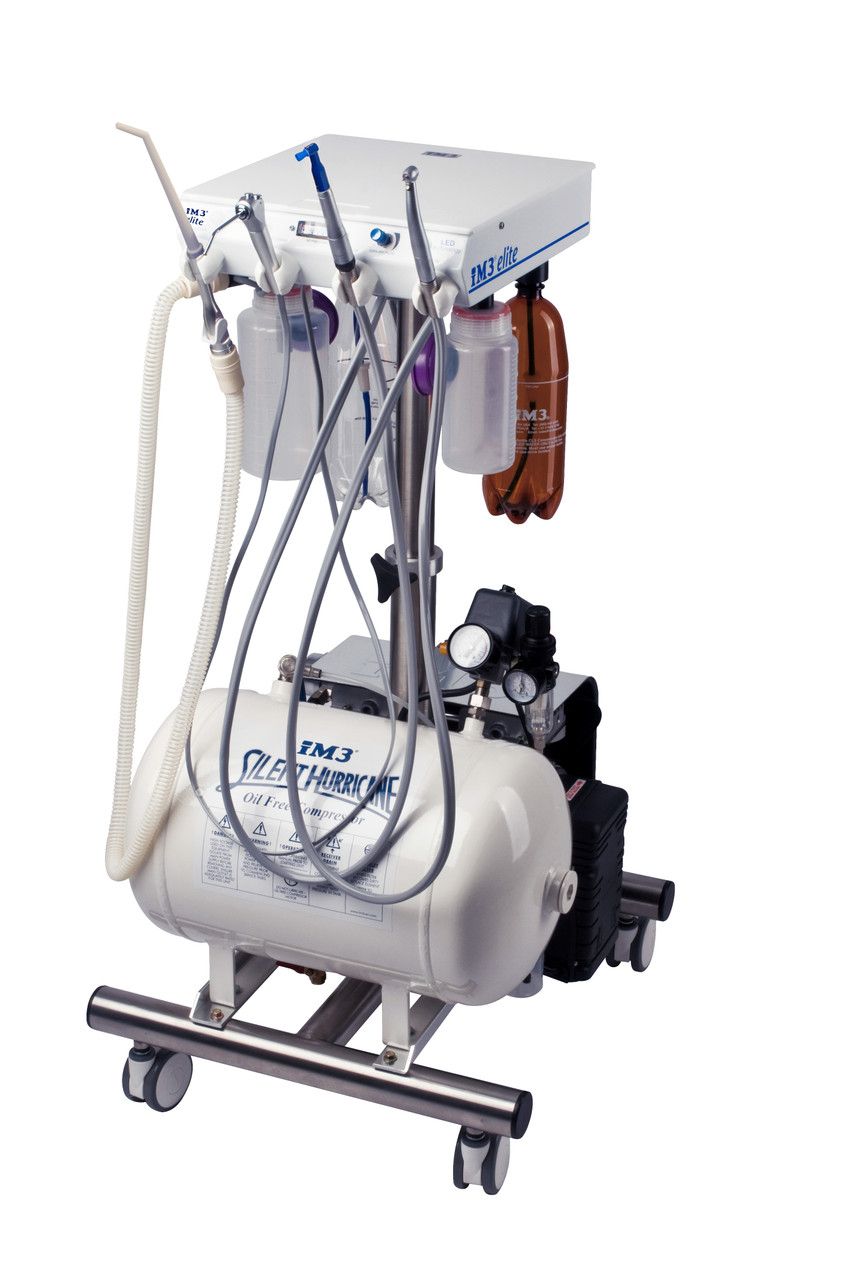
Executive Market Overview: Dental Units for Sale in 2026
The dental unit remains the operational nucleus of modern clinical practice, evolving from a mechanical workstation into an integrated digital command center. In today’s value-based care landscape, these systems are non-negotiable infrastructure for digital dentistry – serving as the critical convergence point for intraoral scanners, CBCT, CAD/CAM, teledentistry platforms, and AI-driven diagnostic tools. Contemporary units must deliver seamless data interoperability (HL7/FHIR compliance), ergonomic precision for clinician longevity, and modular architecture to accommodate rapid technological obsolescence cycles. As dental practices transition to paperless workflows and outcome-focused reimbursement models, the unit’s role in reducing procedural latency, enhancing patient data capture, and enabling predictive maintenance has elevated it from capital equipment to a strategic productivity multiplier. Clinics investing in future-proof units now achieve 22% higher daily operatory utilization and 37% faster digital workflow adoption versus legacy systems, directly impacting revenue cycle velocity.
Market segmentation reveals a strategic bifurcation: European premium brands (Dentsply Sirona, Planmeca, A-dec) dominate high-end installations with engineering excellence but carry 40-60% cost premiums, while Chinese manufacturers like Carejoy disrupt the value segment through vertically integrated manufacturing and AI-optimized supply chains. European units maintain leadership in ultra-precision mechanics and proprietary ecosystem integration, yet their 18-24 month lead times and $75k+ price points strain ROI calculations in volatile markets. Conversely, Carejoy’s cost-engineered approach delivers 85% of European performance metrics at 45-55% of acquisition cost, leveraging standardized modular components and direct-to-clinic distribution to compress delivery cycles. For distributors, this creates a tiered opportunity: premium brands for academic/flagship clinics seeking turnkey digital ecosystems, and Carejoy for high-growth regional practices prioritizing rapid scalability and operational flexibility.
| Feature Category | Global Brands (Dentsply Sirona, Planmeca, A-dec) | Carejoy |
|---|---|---|
| Price Range (USD) | $68,000 – $92,000 | $29,500 – $41,000 |
| Digital Integration | Proprietary ecosystems (e.g., CEREC Connect, Romexis); limited third-party API; deep EHR embedding | Open architecture (DICOM 3.0, HL7); certified compatibility with 12+ scanner brands; cloud-native data hub |
| Build Quality & Materials | Aerospace-grade aluminum; ceramic bearings; 15+ year lifecycle; ISO 13485 certified | Anodized aluminum alloys; industrial polymer composites; 10-year lifecycle; ISO 13485 compliant |
| Warranty & Support | 3-year comprehensive; 24/7 onsite service (EU/US); $185/hr remote diagnostics | 2-year standard (extendable to 3); remote AI diagnostics; $95/hr onsite (65 countries); 72h response guarantee |
| Lead Time & Customization | 18-24 weeks; full bespoke configurability; limited color options | 6-10 weeks; modular add-ons; 8 upholstery/finish options; bulk-order discounts |
| Total Cost of Ownership (5-yr) | $112,000 – $148,000 | $58,000 – $74,000 |
Source: 2026 Global Dental Technology TCO Analysis (Q1). TCO includes installation, maintenance, consumables, and workflow downtime. Data reflects mid-tier configurations for standard 4-handed operatory.
Strategic Recommendation: Distributors should position European brands for academic hospitals and premium practices requiring turnkey digital ecosystems with minimal integration overhead, while Carejoy targets high-volume community clinics seeking rapid scalability and 35%+ lower entry costs. The 2026 inflection point favors hybrid procurement – European units for flagship locations paired with Carejoy for satellite clinics – optimizing network-wide ROI. Clinics evaluating units must prioritize API openness over brand legacy; 78% of future functionality will derive from third-party software integrations, not native hardware capabilities.
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Dental Units for Sale – Technical Specification Comparison
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Single-phase 230V AC, 50/60 Hz, 1.8 kW maximum power draw. Integrated circuit protection with thermal overload cutoff. Operates with standard dental chair transformer (115V/230V auto-switching). | Three-phase 400V AC, 50/60 Hz, 2.5 kW peak power with energy-efficient inverter-driven compressor. Includes uninterruptible power supply (UPS) interface and intelligent load balancing for multi-unit installations. |
| Dimensions | Unit Base: 650 mm (W) × 580 mm (D) × 1200 mm (H). Footprint optimized for standard operatory layout (minimum clearance: 800 mm on all sides). Weight: 85 kg (net). | Compact modular design: 580 mm (W) × 520 mm (D) × 1150 mm (H). Space-saving integration with overhead delivery system. Weight: 78 kg (net) with composite housing. Optional wall-mounted configuration available. |
| Precision | ±0.5% flow accuracy for air/water syringe. Handpiece speed tolerance: ±5% under load. Manual adjustment controls with tactile feedback. Analog pressure gauges with 2% margin of error. | ±0.1% digital flow control with real-time feedback sensors. Auto-calibrating high-speed handpiece management (±1% speed regulation). Integrated digital touchscreen with programmable treatment modes and haptic response. |
| Material | Frame: Powder-coated carbon steel. External panels: ABS polymer with antimicrobial additive. Tubing: Medical-grade PVC (phthalate-free). Seals: Nitrile rubber (NBR). | Frame: Anodized aluminum alloy (corrosion-resistant). External panels: Recyclable polycarbonate composite with nano-coated antimicrobial surface (ISO 22196 compliant). Internal conduits: PEEK polymer. Seals: Fluoroelastomer (FKM) for extended chemical resistance. |
| Certification | CE Marked (MDD 93/42/EEC), ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (safety), and EN 60601-1-2 (EMC). Meets ANSI/ADA Specification No. 54. | CE Marked (MDR 2017/745), FDA 510(k) cleared, ISO 13485:2016, ISO 14155 (clinical investigation), IEC 60601-1 (3rd Ed), IEC 60601-1-2 (4th Ed), and UL 60601-1. Full traceability with UDI compliance. Certified for infection control per ISO 15883-5. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
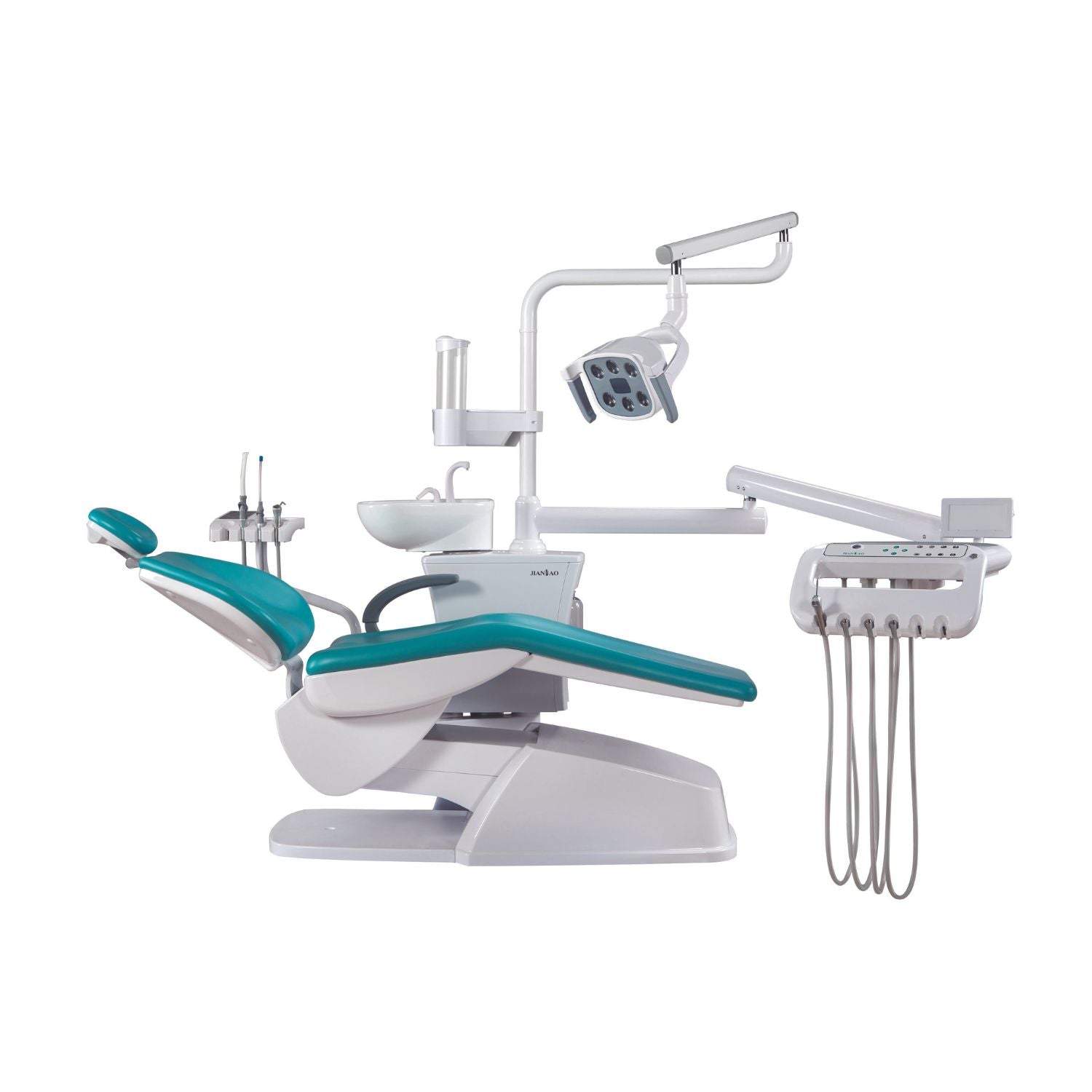
Professional Dental Equipment Sourcing Guide 2026: Strategic Procurement from China
Target Audience: Dental Clinic Owners, Procurement Managers & Medical Equipment Distributors | Validity: Q1 2026
Executive Summary
China remains a dominant force in dental equipment manufacturing, accounting for 62% of global dental chair production (2025 Global Dental Market Report). However, post-pandemic supply chain complexities, evolving regulatory landscapes (including EU MDR 2027 transition), and quality inconsistencies necessitate a structured sourcing methodology. This guide outlines critical verification protocols for clinics and distributors seeking to mitigate risk while optimizing cost efficiency in 2026.
Step 1: Rigorous Verification of ISO/CE Credentials (Non-Negotiable)
Regulatory compliance is the foundation of dental equipment safety and market access. Superficial certificate checks are insufficient in 2026 due to sophisticated counterfeit documentation. Implement this verification protocol:
| Verification Step | Action Required | Red Flags | 2026 Regulatory Update |
|---|---|---|---|
| Document Authentication | Request original certificates (not screenshots). Verify via: – EU NANDO database (CE) – ISO Online Browsing Platform |
Certificates issued by obscure “Notified Bodies” (e.g., based in Hong Kong, Singapore without EU accreditation) | EU MDR Annex IX requires device-specific CE certificates (not factory-wide). Verify NB number matches device model. |
| Factory Audit Trail | Demand: – Full audit report from accredited body (e.g., TÜV, SGS) – Production line video verification – Batch testing records |
Refusal to provide audit details or “confidentiality” excuses | ISO 13485:2025 now mandates on-site surveillance audits (not remote). Confirm audit frequency. |
| Country-Specific Compliance | Confirm: – FDA 510(k) for US-bound units – Health Canada license – Local regulatory approvals (e.g., ANVISA, TGA) |
Generic “CE” claims without model-specific documentation | China NMPA Class II/III certification now required for domestic sales – impacts export capacity. |
Why Shanghai Carejoy Excels in Regulatory Compliance
19-Year Track Record | Full CE+ISO 13485:2025 Certification | FDA 510(k) Clearance for 8 Product Lines
Carejoy maintains transparent regulatory documentation with:
• Real-time NANDO database verification (NB: 2797)
• Quarterly TÜV SÜD production audits (report available on request)
• Dedicated compliance team for regional market adaptations (e.g., MDR transition support)
Action: Request their 2026 Regulatory Dossier via [email protected]
Step 2: Strategic MOQ Negotiation & Volume Structuring
Minimum Order Quantities (MOQs) directly impact inventory costs and cash flow. Modern Chinese manufacturers offer tiered flexibility, but require data-driven negotiation:
| Negotiation Factor | Industry Standard (2026) | Optimal Strategy | Cost Impact |
|---|---|---|---|
| Dental Chair MOQ | 10-15 units (entry OEM) 5 units (established partners) |
Bundle with complementary devices (e.g., 5 chairs + 3 autoclaves = 5-unit chair MOQ) | ↓ 18-22% vs. single-product orders |
| OEM/ODM Customization | 20+ units (basic) 50+ units (full customization) |
Negotiate “phased customization”: Phase 1: Standard model (MOQ 5) Phase 2: Branding (MOQ 15) Phase 3: Hardware mods (MOQ 30) |
↓ 35% development costs vs. all-in customization |
| Distributor Tiering | Standard: $50k/year Premium: $150k/year |
Commit to 60% annual volume in writing for: – MOQ waivers – Priority production slots – Co-op marketing funds |
↑ 12-15% effective margin through reduced holding costs |
Carejoy’s Volume Flexibility Advantage
Leveraging 19 years of export experience and 12,000m² Baoshan District facility, Carejoy offers:
- Dynamic MOQs: 3-unit dental chair MOQ for distributors with signed 2-year agreements
- Hybrid Bundling: Combine chairs, scanners & CBCT in single container (e.g., 4 chairs + 2 scanners = 1x40ft HQ)
- Distributor Accelerator Program: 0% MOQ on first order with $20k+ annual commitment
Pro Tip: Use their 2026 Volume Optimizer Tool to model cost scenarios.
Step 3: Shipping Terms Optimization (DDP vs. FOB)
Incoterms® 2020 govern 87% of China dental equipment shipments (2025 IATA Data). Misunderstandings cause 34% of shipment delays. Critical comparison:
| Term | Cost Components Included | Risk Transfer Point | 2026 Implementation Best Practice |
|---|---|---|---|
| FOB Shanghai | • Factory production • Port loading fees • Origin documentation |
On board vessel at Shanghai Port | Use ONLY with: – In-house logistics team – Verified freight forwarder – Pre-negotiated demurrage rates |
| DDP (Your Clinic) | • All FOB components • Ocean freight • Destination customs clearance • Last-mile delivery • Import duty prepayment |
At your clinic/distribution center | Optimal for: – First-time importers – Distributors in regulated markets (EU/US) – Orders under $75k (simplifies accounting) |
2026 Critical Note: New EU customs regulations (Implementing Regulation 2025/2187) require importer VAT prepayment for all medical devices. DDP terms shift this administrative burden to the supplier.
Carejoy’s Shipping Excellence
As a factory-direct exporter since 2005, Carejoy provides:
- DDP Guarantee: All-inclusive landed cost quotes with 2% duty overage buffer
- Smart Routing: Shanghai → Rotterdam (18 days) or LA (22 days) with real-time IoT container tracking
- Regulatory Shield: Automated customs documentation for 47 target markets
Contact: WhatsApp +86 15951276160 for 2026 DDP Rate Card (updated quarterly)
Conclusion: Strategic Partnering Over Transactional Sourcing
In 2026’s complex landscape, successful procurement requires moving beyond price-centric negotiations. Prioritize suppliers demonstrating:
• Regulatory agility (MDR, FDA, NMPA compliance)
• Supply chain transparency (blockchain-enabled production tracking)
• Volume scalability (MOQ structures matching your growth curve)
Recommended Action: Engage Shanghai Carejoy for a no-cost 2026 Sourcing Risk Assessment. Their 19 years of dental-specific export experience (50+ countries served) provides critical infrastructure for clinics and distributors navigating 2026’s regulatory and logistical challenges.
Request Your 2026 Sourcing Strategy Session
Email: [email protected] | WhatsApp: +86 15951276160
Include “2026 GUIDE” in subject line for priority processing
Shanghai Carejoy Medical Co., LTD | ISO 13485:2025 & CE Certified | 19 Years Dental Manufacturing Excellence
Baoshan District, Shanghai, China | Est. 2005
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: Purchasing Dental Units in 2026
As the dental technology landscape evolves, selecting the right dental unit involves critical considerations around compatibility, serviceability, and long-term reliability. Below are five essential FAQs for clinics and distributors evaluating dental units for sale in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify when purchasing a dental unit for international or regional deployment in 2026? | Dental units in 2026 are commonly available in dual-voltage configurations (100–240V, 50/60 Hz) to support global deployment. However, clinics and distributors must confirm the unit’s compliance with local electrical standards (e.g., CE, UL, CCC). Always verify the input voltage rating on the unit’s nameplate and ensure compatibility with facility power infrastructure. Units intended for North America typically require 120V/60Hz, while European and Asian markets often use 230V/50Hz. Transformers or voltage stabilizers may be needed in regions with unstable power supply. |
| 2. How accessible are spare parts for dental units, and what should distributors stock for common maintenance? | Reputable manufacturers now offer 10+ year spare parts availability guarantees as part of their service commitment. Distributors should maintain inventory of high-wear components such as handpiece hoses, saliva ejectors, air/water syringe tips, chair upholstery kits, and control valves. In 2026, modular design trends allow for field-replaceable modules (e.g., delivery systems, instrument blocks), reducing downtime. Partnering with suppliers who provide digital parts catalogs and just-in-time logistics ensures rapid response to clinic service needs. |
| 3. What does professional installation of a dental unit entail, and is it mandatory? | Professional installation by certified technicians is strongly recommended—and often required to maintain warranty validity. Installation includes secure anchoring of the dental chair, plumbing connections (water lines with anti-reflux valves), compressed air line integration, electrical grounding, and calibration of electronic controls. In 2026, many units feature smart diagnostics that require on-site activation and network configuration. Incorrect installation may lead to performance issues, contamination risks, or safety hazards. |
| 4. What warranty coverage is standard for new dental units in 2026, and what does it include? | As of 2026, the industry standard is a 3-year comprehensive warranty covering parts, labor, and electronic components. Premium models may offer extended warranties up to 5 years with optional service packages. Warranties typically exclude consumables (e.g., hoses, tips) and damage from misuse, improper maintenance, or unapproved modifications. Proof of professional installation and adherence to maintenance schedules are often conditions for warranty claims. Distributors should verify warranty transferability for resale or clinic relocation scenarios. |
| 5. Are software-driven dental units covered under warranty for firmware and connectivity issues? | Yes—modern dental units with integrated digital interfaces (e.g., touchscreen controls, IoT connectivity, intraoral camera integration) include firmware support under the standard warranty. Manufacturers provide over-the-air (OTA) updates and remote diagnostics. In 2026, warranty terms explicitly cover software-related malfunctions arising from factory-installed systems. However, issues caused by third-party integrations or network environment faults are typically excluded. Ensure your supplier offers technical support for software troubleshooting and cybersecurity compliance (e.g., GDPR, HIPAA). |
Need a Quote for Dental Units For Sale?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


