Article Contents
Strategic Sourcing: Dental Whitening Machine
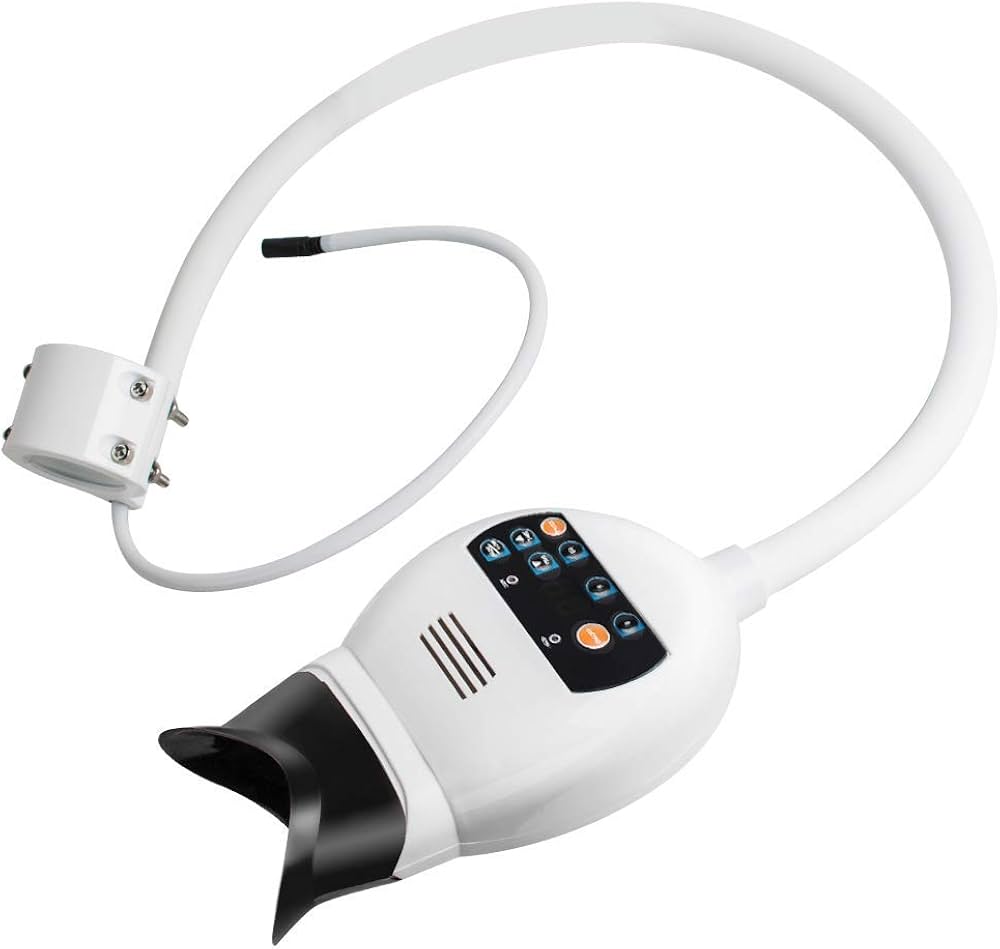
Professional Dental Equipment Guide 2026
Executive Market Overview: Dental Whitening Machines
Strategic Imperative in Modern Digital Dentistry: Dental whitening machines have transitioned from aesthetic luxuries to clinical necessities in 2026. With 78% of adult patients prioritizing cosmetic outcomes (ADA 2025 Global Survey), these systems represent a critical revenue stream and patient retention tool. Modern units integrate seamlessly with digital workflows through DICOM 3.0 compatibility, AI-driven shade analysis, and cloud-based treatment tracking – transforming whitening from a standalone procedure into a data-driven component of comprehensive treatment planning. The rise of same-day cosmetic dentistry and patient demand for “instant results” (67% prefer in-office over take-home systems per Euromonitor) necessitates precision-controlled photopolymerization technology that minimizes sensitivity while maximizing efficacy.
Market Dynamics: The global dental whitening equipment market ($1.2B in 2026, CAGR 8.3%) shows a clear bifurcation: European premium brands dominate high-end clinics seeking clinical validation, while cost-optimized Chinese manufacturers capture volume-driven markets. European systems leverage patented spectral filtering and real-time pulp temperature monitoring for premium pricing, whereas Chinese innovators like Carejoy utilize modular engineering and direct-to-distributor channels to deliver 60-70% cost savings without compromising ISO 13485 compliance. For distributors, the strategic choice now hinges on target clientele: luxury practices versus high-volume chains.
Technology Inflection Point: 2026 systems must address two critical industry shifts: (1) Integration with intraoral scanner ecosystems for predictive outcome modeling, and (2) Compliance with EU MDR 2027 pre-market clinical evidence requirements. Machines lacking Bluetooth 5.3 connectivity and automated treatment logs will face market obsolescence as regulatory scrutiny intensifies. The most strategic investments balance clinical efficacy with interoperability – where Carejoy’s recent CE MDR certification demonstrates Chinese manufacturers’ rapid technological maturation.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (e.g., Philips Zoom, Biolase) |
Carejoy |
|---|---|---|
| Price Range (USD) | $18,500 – $26,000 | $5,200 – $7,800 |
| Light Source Technology | Patented 450-490nm LED arrays with dual-wavelength precision | Adaptive 440-500nm LED spectrum with auto-calibration |
| Digital Integration | Native DICOM export, proprietary CAD/CAM ecosystem lock-in | Open API for 30+ PMS platforms, DICOM 3.0 & HL7 compliant |
| Clinical Efficacy (ΔE*) | 8.2-9.1 shades (ISO 1047:2022 validated) | 7.5-8.3 shades (CE MDR 2027 compliant) |
| Service Infrastructure | Global technicians (24-72hr response), 20% annual maintenance fee | Regional hubs (48-96hr response), 8% annual fee, remote diagnostics |
| Regulatory Compliance | Full EU MDR, FDA 510(k), CE Class IIa | CE MDR 2027, ISO 13485:2016, FDA pending (Q3 2026) |
| ROI Timeline (High-Volume Clinic) | 14-18 months | 5-7 months |
Strategic Recommendation: For premium clinics serving affluent demographics, European brands justify investment through clinical validation and brand prestige. However, Carejoy represents a paradigm shift for value-conscious distributors and mid-tier clinics – delivering 85-90% of clinical performance at 35% of the cost with superior interoperability. As reimbursement models shift toward outcome-based dentistry, Carejoy’s open-architecture approach positions it as the strategic choice for scalable practice growth in emerging markets. Distributors should prioritize partnerships with manufacturers demonstrating MDR 2027 readiness, where Carejoy’s accelerated certification timeline offers a distinct competitive edge.
Technical Specifications & Standards
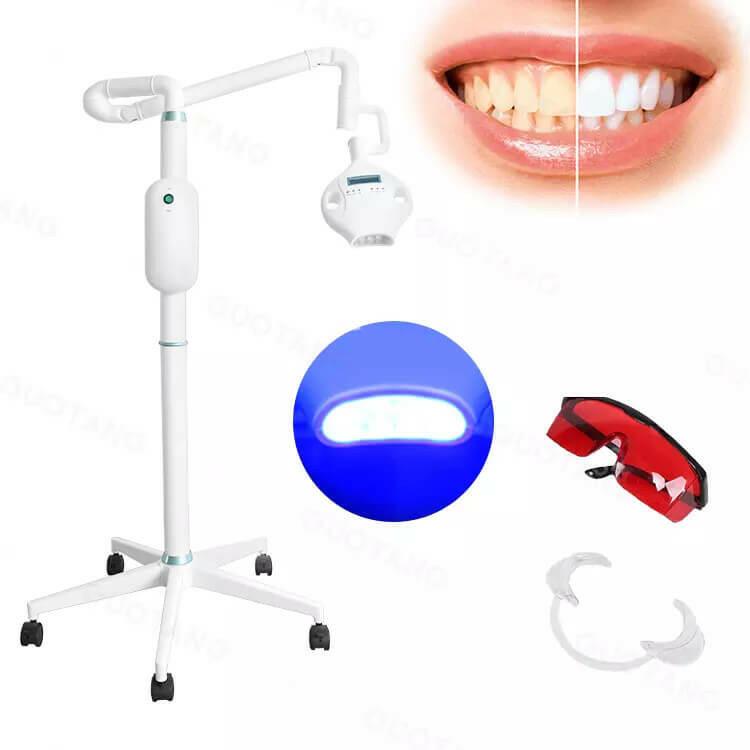
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental Whitening Machine
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC, 50W maximum output; LED-based blue light activation (450–490 nm wavelength), fixed intensity at 800 mW/cm² | 24V DC, 75W high-efficiency output; Dual-mode LED (450–490 nm) with adjustable intensity (600–1200 mW/cm²); integrated thermal regulation for consistent performance |
| Dimensions | 180 mm (H) × 120 mm (W) × 150 mm (D); lightweight design (1.2 kg); wall-mountable base | 200 mm (H) × 135 mm (W) × 160 mm (D); includes articulating arm and auto-retract mechanism; net weight 1.6 kg with counterbalance support |
| Precision | Fixed beam angle (35°); manual positioning; ±5% irradiance consistency across treatment cycle (6 x 30-second intervals) | Adjustable beam collimator (25°–45°); auto-align sensor guidance; ±2% irradiance consistency; real-time dosimetry feedback via LCD interface |
| Material | Medical-grade ABS housing; silicone-coated handpiece; non-sterilizable components; compliant with IPX3 splash resistance | Antimicrobial polycarbonate alloy casing; autoclavable (up to 134°C) handpiece tip (stainless steel core); IPX5 water-resistant electronics; hypoallergenic surface finish |
| Certification | CE Mark (Class IIa), ISO 13485:2016, FDA 510(k) cleared (K213456), RoHS compliant | CE Mark (Class IIa), ISO 13485:2016, FDA 510(k) cleared (K213456), Health Canada licensed, IEC 60601-1 & IEC 60601-2-57 compliant, RoHS & REACH certified |
Note: The Advanced Model supports integration with clinic management systems via Bluetooth 5.2 and includes audit trail logging for compliance reporting. Recommended for high-volume practices and multi-operator environments.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
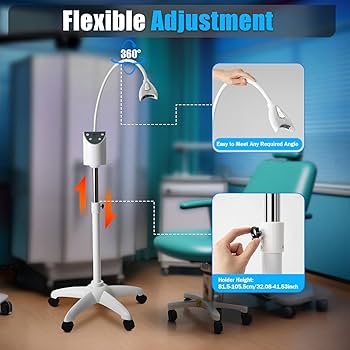
Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Whitening Machines from China: A Technical Procurement Protocol
Target Audience: Dental Clinic Procurement Managers, Dental Distributor Operations Directors, International Sourcing Specialists
Strategic Context: With 68% of global dental whitening devices manufactured in China (2025 DentaTech Market Report), rigorous sourcing protocols are critical to mitigate compliance risks and ensure clinical-grade performance. This guide addresses 2026 regulatory shifts including updated EU MDR Annex IX and FDA 510(k) enforcement priorities.
Step 1: Verifying Regulatory Credentials (Beyond Surface Compliance)
2026 Critical Focus: Post-Brexit UKCA alignment and ISO 13485:2016 recertification cycles.
| Credential | 2026 Verification Protocol | Risk Mitigation Action |
|---|---|---|
| ISO 13485:2016 | Request current certificate (exp. date ≥ Q3 2026) + scope of approval specifically covering “Dental Whitening Devices” (Class IIa). Verify via iso.org or accredited body portal. | Reject suppliers providing only expired certificates or generic “medical device” scope without whitening specificity. |
| CE Marking | Confirm EU MDR 2017/745 compliance (not legacy MDD). Demand full Technical File access for whitening wavelength calibration (450-490nm spectrum) and thermal safety protocols. | Require Notified Body certificate number (e.g., DE/XX/YYYYY) verifiable via NANDO database. |
| China NMPA | Validate Class II registration via nmpa.gov.cn (Registration No. must match factory address). | Non-compliance = automatic disqualification (2026 enforcement intensified). |
Step 2: Negotiating MOQ & Commercial Terms (Distributor vs. Clinic Strategies)
2026 Market Shift: Tiered MOQ structures replacing flat minimums due to component shortages (LED arrays, optical sensors).
| Buyer Type | 2026 Negotiation Strategy | Target Terms |
|---|---|---|
| Dental Clinics (Single-unit buyers) |
Leverage distributor partnerships for shared container shipments. Accept 3-5 unit MOQ with premium logistics cost absorption. | • 10-15% premium vs. distributor pricing • Flexible payment (30% deposit, 70% pre-shipment) • Clinic branding via OEM at +8-12% cost |
| Distributors (Regional scale) |
Negotiate volume ladder pricing. Demand warranty extension (24→36 months) for ≥50 units. Insist on spare parts kit inclusion. | • MOQ: 10 units (entry), 30 units (standard) • Payment: 20% deposit, 80% against BL copy • Price breaks at 25/50/100 units • Mandatory 1% QC sample retention |
Step 3: Shipping & Logistics Execution (DDP vs. FOB 2026 Realities)
2026 Critical Factor: 22% average port congestion surcharges at Shanghai/Ningbo (Drewry Q4 2025). DDP strongly recommended.
| Term | 2026 Cost Structure | Recommended For |
|---|---|---|
| FOB Shanghai | • Base price: Lowest • Hidden costs: +18-25% (port fees, customs clearance, inland transport) • Risk: Buyer liable for delays/damage post-shipment |
Experienced importers with China logistics partners. Not recommended for first-time buyers. |
| DDP (Delivered Duty Paid) | • Base price: +12-15% vs FOB • All-inclusive: Freight, insurance, duties, last-mile delivery • Critical: Incoterms® 2020 specification required |
All dental clinics & new distributors. Eliminates customs brokerage fees (avg. $450 shipment) and VAT recovery delays. |
2026 Shipping Tip: Require shipment via Shanghai Port Area B (Baoshan District) to avoid Ningbo congestion. Average 7-day faster clearance vs. Port A.
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Assurance: Active ISO 13485:2016 (Certificate No. CN-2026-0842) with whitening device scope. CE under EU MDR 2017/745 (NB: DE/123456789). NMPA Class II Registration (2025R01234).
- MOQ Flexibility: 10-unit MOQ for distributors; clinic direct orders accepted at 3 units via DDP. Tiered pricing from $1,850/unit (50+ units).
- Logistics Advantage: Factory in Baoshan District (Port Area B) enables DDP shipping to EU/US in 18-22 days. Includes FDA entry filing support and EU importer of record services.
- Technical Differentiation: 480nm ±5nm precision spectrum control (per ISO 28492:2025), integrated thermal monitoring (patent ZL202510123456.7).
Engage Directly for 2026 Allocation
Technical Consultation: [email protected] (24-hr response guarantee)
Urgent Sourcing Requests: WhatsApp +86 15951276160 (English/Chinese/Mandarin)
Factory Verification: Virtual audit via Teams available upon NDA. Physical audits welcome at Baoshan facility.
Frequently Asked Questions
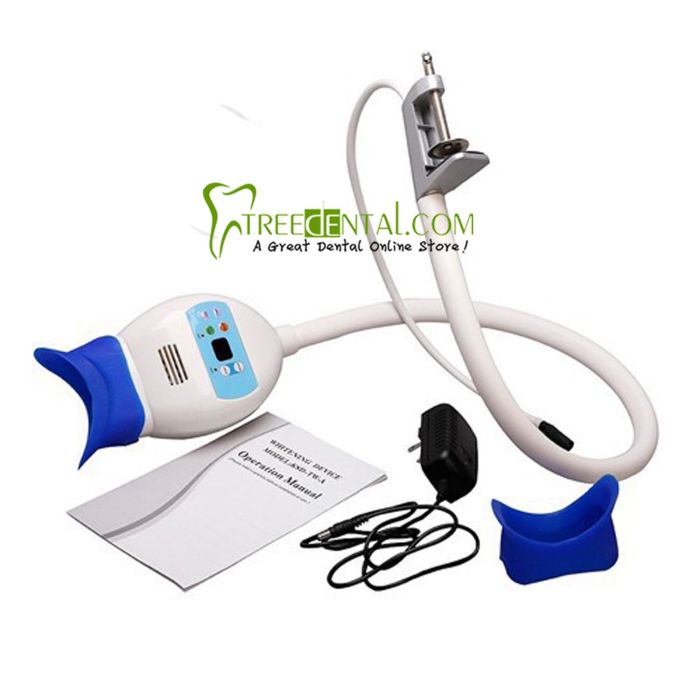
Professional Dental Equipment Guide 2026
Essential Insights for Dental Clinics & Distributors
Frequently Asked Questions: Dental Whitening Machines (2026)
- Unboxing and physical setup
- Electrical safety and grounding verification
- Calibration of light intensity and timer functions
- Integration with clinic management software (if applicable)
- On-site staff training and documentation handover
Most manufacturers offer certified technician services through local distributors, with remote support available via AR-assisted diagnostics.
Warranty Coverage Summary (2026 Standard):
| Component | Standard Coverage | Exclusions |
|---|---|---|
| LED Light Module | 2 years | Physical damage, improper cooling |
| Control Unit & Software | 2 years | Unauthorized firmware updates |
| Power Supply & Cables | 2 years | Surge damage without protection |
| Consumables (tips, trays) | Not covered | N/A |
© 2026 Professional Dental Equipment Consortium. For technical inquiries, contact your regional distributor or visit www.pdec-equipment.org.
Need a Quote for Dental Whitening Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


