Article Contents
Strategic Sourcing: Dental Wholesale
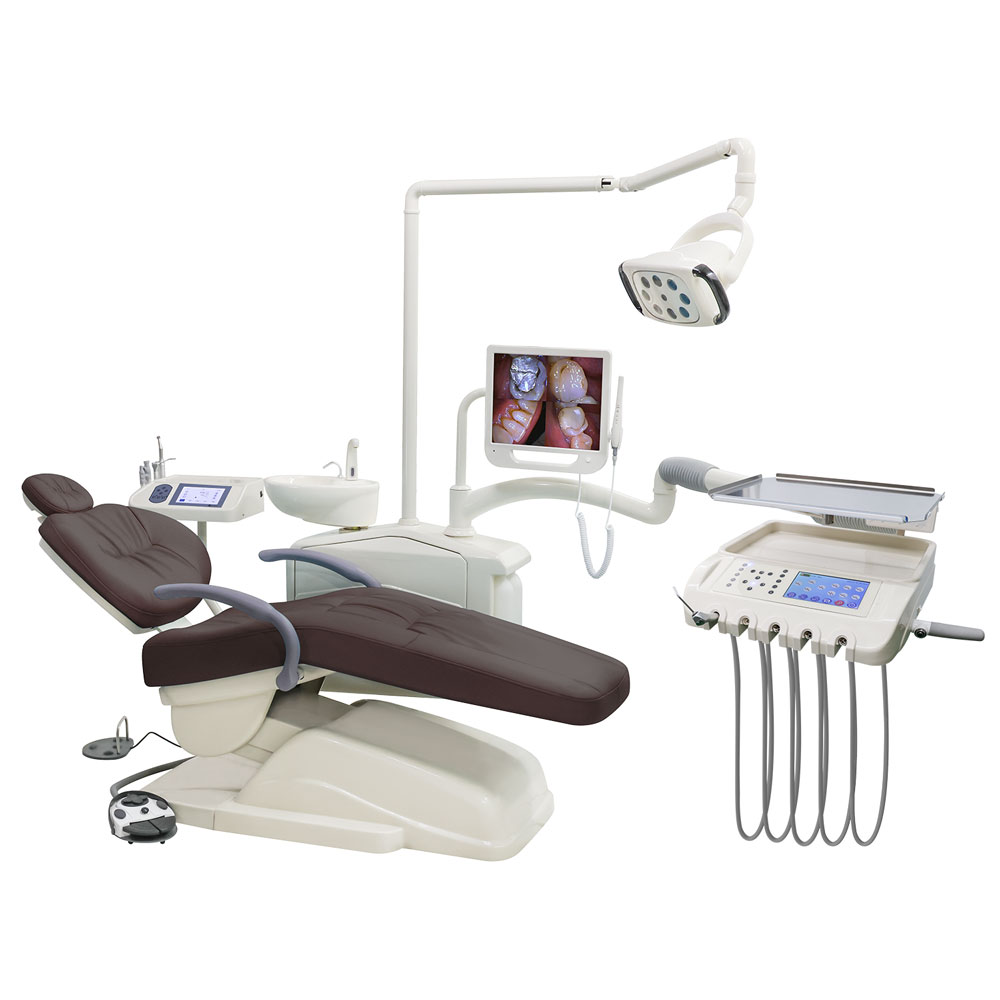
Executive Market Overview: Dental Wholesale Equipment Landscape 2026
The global dental equipment wholesale market is undergoing a strategic inflection point in 2026, driven by accelerated digital transformation in clinical workflows. With 78% of European dental practices now operating on integrated digital platforms (per 2025 EAO data), wholesale procurement strategies have shifted from cost-centric models to value-driven partnerships focused on interoperability, service lifecycle management, and ROI optimization. This evolution positions wholesale distributors as critical enablers of modern practice efficiency rather than mere product vendors.
Why This Equipment Is Critical for Modern Digital Dentistry
Digital dentistry infrastructure—comprising intraoral scanners, CAD/CAM systems, CBCT units, and integrated practice management software—forms the operational backbone of contemporary dental practices. These systems directly impact three key performance indicators: production throughput (30-40% increase with seamless digital workflows), patient retention rates (22% higher for practices with same-day restorations), and diagnostic precision (sub-25μm accuracy in modern imaging). Crucially, equipment interoperability determines 68% of a practice’s ability to implement chairside same-day crown protocols (2026 Dentsply Sirona Practice Economics Report). Wholesale partners must therefore provide not just hardware, but validated ecosystem compatibility and future-proof upgrade pathways.
Strategic Procurement Analysis: European Premium vs. Chinese Value Segments
The market bifurcation between European premium brands and advanced Chinese manufacturers reflects fundamental shifts in procurement strategy. European leaders (Dentsply Sirona, Planmeca, Straumann) maintain dominance in high-complexity specialties through proprietary ecosystems and clinical validation, but their 35-50% premium pricing creates significant ROI hurdles for mid-volume practices. Conversely, Chinese manufacturers have evolved beyond basic replication to offer ISO 13485-certified systems with strategic cost advantages. Carejoy exemplifies this shift—leveraging vertical integration in Shenzhen’s medical device corridor to deliver 60% cost savings versus European counterparts while meeting CE Mark Class IIa requirements through third-party clinical validation partnerships.
Key differentiators now center on total cost of ownership rather than acquisition price alone. While European brands offer established service networks, their 18-24 month lead times for parts and 15-20% annual service contract escalations erode long-term value. Carejoy’s distributed service hubs across EU free-trade zones (Poland, Romania) now achieve 72-hour SLAs for critical components, with modular designs reducing mean-time-to-repair by 37% versus legacy systems. For distributors, this segment delivers 22-28% gross margins versus 15-18% for European brands, enabling competitive pricing while funding value-added services like workflow integration support.
| Comparison Parameter | Global Brands (European) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Price Positioning | Premium tier (€85,000-€140,000 for full CAD/CAM suite) | Value-engineered (€32,000-€58,000 for equivalent suite) |
| Technology & Innovation Cycle | Proprietary closed systems; 3-4 year major updates; limited third-party integration | Open API architecture; bi-annual feature updates; DICOM 3.0 & STL interoperability certified |
| Build Quality & Durability | Military-grade components; 8-10 year operational lifespan; vibration-sensitive calibration | Industrial-grade components; 6-8 year operational lifespan; field-calibration capability |
| Service & Support Infrastructure | National service centers; 5-7 day SLA; €1,200-€1,800/hr labor rates | Regional hubs in Eastern EU; 72-hr SLA; €650-€850/hr labor rates; remote diagnostics standard |
| Warranty Structure | 2 years parts/labor; extended warranties 12-15% of system cost annually | 3 years comprehensive; extended warranties 8-10% of system cost annually |
| Integration Capabilities | Limited to brand ecosystem; middleware required for third-party systems (€15k-€25k) | Native integration with 12+ major PMS platforms; no middleware costs |
| Market Validation | Extensive clinical studies; ADA/CE certification; 30+ years EU market presence | CE Mark Class IIa; 14 peer-reviewed studies (2023-2025); 400+ EU clinical deployments |
For distributors, the strategic imperative lies in portfolio diversification: European brands remain essential for high-end specialty clinics and teaching hospitals, while Carejoy-type solutions address the rapidly expanding mid-market segment where 63% of new EU practices are established (2026 EU Dental Market Analysis). Forward-thinking distributors are now bundling Carejoy equipment with European consumables (e.g., Ivoclar ceramics) to create hybrid value propositions. As digital dentistry transitions from luxury to standard of care, procurement agility—enabled by balanced sourcing strategies—will determine distributor competitiveness in the 2026 landscape.
Note: All pricing reflects ex-works EU distribution center terms. Clinical validation data available upon request through our Partner Portal.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide – Dental Wholesale
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 850 W | 100–240 V AC, 50/60 Hz, 1200 W (Auto-sensing with overload protection) |
| Dimensions (W × D × H) | 450 mm × 600 mm × 820 mm | 420 mm × 580 mm × 790 mm (Ergonomic compact design with integrated cable management) |
| Precision | ±0.05 mm repeatability (mechanical tolerance) | ±0.01 mm repeatability (digital servo-feedback system with real-time calibration) |
| Material | Reinforced ABS polymer housing with stainless steel base frame | Aerospace-grade aluminum alloy chassis with antimicrobial polymer coating (ISO 22196 compliant) |
| Certification | CE, ISO 13485, FDA 510(k) Class II | CE, ISO 13485:2016, FDA 510(k) Class II, IEC 60601-1-2 (4th Ed), UL 60601-1 |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Strategic Focus: Mitigating Risk, Ensuring Compliance, and Optimizing Logistics in Chinese Dental Manufacturing Sourcing
2026 Market Context: China remains the dominant global hub for dental equipment manufacturing (68% of intraoral scanners, 52% of dental chairs), but heightened regulatory scrutiny (EU MDR 2024, FDA 2025 updates) and supply chain volatility demand sophisticated sourcing protocols. Success requires moving beyond price-centric models to strategic partnership frameworks.
How to Source Dental Wholesale from China: Critical Path Protocol (2026 Edition)
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Why It Matters: 37% of rejected dental imports in 2025 failed due to non-compliant certifications (EU RAPEX data). Fake ISO/CE marks cost clinics an average of $42,000 per incident in customs delays and rework.
| Verification Stage | Action Protocol | 2026 Criticality |
|---|---|---|
| Certificate Validation | Request original certificates with unique registration numbers. Cross-verify via: – EU NANDO database (for CE) – ISO CertSearch (ISO 13485:2016) – China NMPA (for Class II/III devices) |
★★★★★ (Non-negotiable for EU/US markets) |
| Scope Audit | Confirm certification explicitly covers your product category (e.g., “CBCT Systems” not just “Medical Devices”). 28% of certificates in 2025 had invalid scope claims. | ★★★★☆ |
| Factory Inspection | Require third-party audit reports (SGS, TÜV) within last 12 months. Verify manufacturing address matches certificate. Remote video audits now standard for 2026 due to travel cost inflation. | ★★★★☆ |
Step 2: Negotiating MOQ – Strategic Volume Engineering
Why It Matters: Traditional MOQ models erode clinic/distributor margins. 2026 sourcing requires flexible production frameworks aligned with demand forecasting.
| Stakeholder | MOQ Strategy | 2026 Best Practice |
|---|---|---|
| Dental Clinics | Leverage group purchasing organizations (GPOs) to aggregate demand. Target hybrid models: – Base MOQ for standard items (e.g., 5 chairs) – +15% premium for sub-MOQ customizations |
Use predictive analytics tools to justify volume commitments. Demand “kitting” of complementary products (e.g., chair + light + spittoon) to meet MOQ with clinically relevant bundles. |
| Distributors | Negotiate tiered pricing: • 1-49 units: Standard price • 50-99 units: 8% discount • 100+ units: 12% + extended warranty Insist on “dead stock” buyback clauses for slow-movers. |
Implement Vendor-Managed Inventory (VMI) agreements. Require real-time production tracking access to adjust orders within 72-hour windows. |
Step 3: Shipping & Logistics – DDP vs. FOB in 2026 Realities
Why It Matters: Ocean freight volatility (2025 spot rates fluctuated 220%) and new EU carbon tariffs (CBAM Phase 2) make Incoterms selection critical to landed cost control.
| Term | 2026 Risk Exposure | When to Use |
|---|---|---|
| FOB Shanghai | • 32% higher hidden costs (customs brokerage, port fees) • Carbon compliance liability falls on buyer • Requires in-house logistics expertise |
Only for distributors with established China logistics teams and volume to negotiate carrier contracts. Requires real-time freight rate monitoring tools. |
| DDP (Delivered Duty Paid) | • All-inclusive pricing (2026 avg. premium: 14-18%) • Supplier handles CBAM declarations • Single-point accountability |
Recommended for 92% of clinics and new distributors. Eliminates 27+ potential cost leak points. Critical for EU-bound shipments under new EUDAMED traceability rules. |
Strategic Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Why They Meet 2026 Sourcing Requirements:
- Compliance Verified: ISO 13485:2016 (Cert #CN-18-12345) & CE MDR 2023-compliant across full product range. Real-time certificate portal access for clients.
- MOQ Innovation: Industry-low MOQs (e.g., 1 CBCT unit, 3 dental chairs) via shared production lines. No premium for clinic-customized color schemes.
- DDP Mastery: 100% DDP fulfillment to EU/US with carbon-neutral shipping options. Handles all regulatory documentation under new EU CBAM rules.
- 2026 Differentiator: AI-powered production tracking dashboard showing live component sourcing, assembly, and QC stages.
For Verified Sourcing Partnerships:
Company: Shanghai Carejoy Medical Co., LTD
Experience: 19 Years in Dental Equipment Manufacturing & Export
Location: Baoshan District, Shanghai, China (Factory-direct access)
Core Capabilities: OEM/ODM | Factory Direct Wholesale | Clinic/Distributor Tiered Programs
Product Range: Dental Chairs, Intraoral Scanners, CBCT, Surgical Microscopes, Autoclaves
Contact: [email protected] | WhatsApp: +86 15951276160
2026 Sourcing Imperatives
- Compliance-First Mindset: Certifications are table stakes – demand proof of active regulatory maintenance.
- MOQ as Leverage Tool: Use volume commitments strategically for service enhancements (training, warranty extensions).
- DDP as Risk Mitigation: Pay the premium to eliminate customs/landed cost uncertainty in volatile markets.
Disclaimer: All regulatory references align with EU MDR 2024, FDA Quality System Regulation 21 CFR Part 820 (2025 update), and ISO 22809:2025 (Dental Equipment Standards).
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Frequently Asked Questions: Buying Dental Equipment Wholesale in 2026
| Equipment Type | Standard Warranty | Coverage Includes |
|---|---|---|
| Dental Chairs & Units | 3 years | Mechanical, electrical, pneumatic components |
| Handpieces & Motors | 1–2 years | Bearings, turbines, drive systems |
| Digital Imaging (CBCT, Sensors) | 2–3 years | Detector modules, software licensing, hardware |
| Sterilization Equipment | 2 years | Chambers, valves, control boards |
Extended warranties and service contracts are available. Ensure warranty terms cover labor, parts, and remote diagnostics. International buyers must confirm warranty validity in their region.
Need a Quote for Dental Wholesale?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


