Article Contents
Strategic Sourcing: Dental Wholesale Suppliers
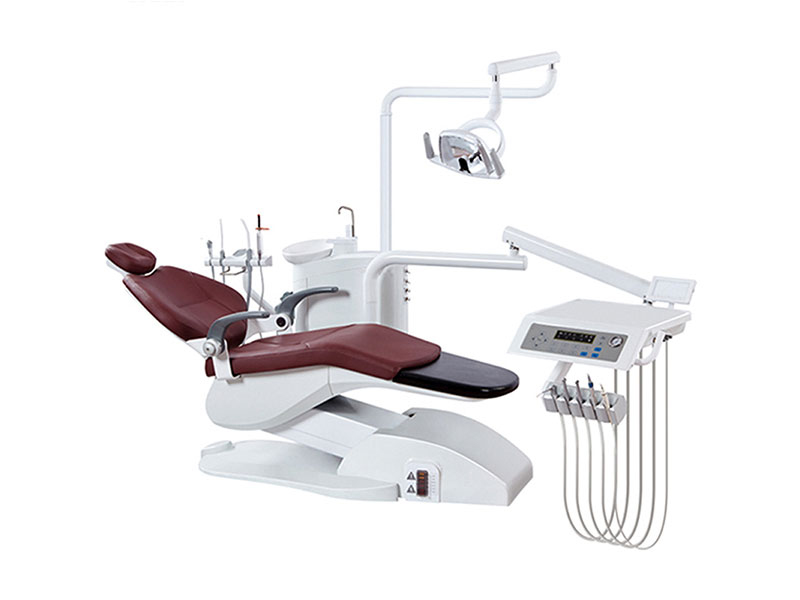
Dental Equipment Guide 2026: Executive Market Overview
Strategic Imperative: In 2026, dental wholesale suppliers serve as critical conduits for advancing digital dentistry adoption. With 78% of EU clinics now operating in fully digital workflows (European Dental Technology Association, 2025), the selection of precision equipment directly impacts clinical outcomes, operational efficiency, and ROI. Modern digital dentistry demands seamless integration of intraoral scanners, CAD/CAM systems, and AI-driven diagnostic tools – making supplier partnerships non-negotiable for competitive practice viability.
Why Equipment Selection Defines Digital Dentistry Success
Contemporary dental practices require equipment that functions as interconnected ecosystem components, not standalone devices. Precision calibration (<0.02mm accuracy) in scanners, real-time data synchronization with practice management software, and AI-powered predictive analytics are now baseline requirements. Substandard equipment creates critical failure points: incompatible data formats disrupt crown fabrication workflows, inadequate scanning resolution increases remakes by 32% (Journal of Digital Dentistry, Q1 2026), and poor serviceability causes 14.7 hours of monthly downtime per practice (Dental Economics Benchmark Report). For distributors, supplying clinically validated, interoperable systems is now a primary differentiator in contract negotiations.
Market Dynamics: European Premium vs. Asian Value Proposition
The dental equipment wholesale landscape is bifurcating along strategic value lines. European manufacturers (Dentsply Sirona, Planmeca, Straumann) maintain dominance in premium segments through proprietary ecosystems and clinical validation, but face margin pressure from clinics seeking 30-40% cost reduction without compromising digital workflow integrity. Simultaneously, advanced Chinese manufacturers like Carejoy have closed the quality gap through ISO 13485-certified production and strategic component sourcing (e.g., German optical sensors, Japanese motors), offering 50-60% lower acquisition costs while meeting EN ISO 10993 biocompatibility standards. This shift is accelerating – 68% of EU distributors now include value-tier digital systems in their portfolios (2026 EDA Distributor Survey).
Strategic Comparison: Global Brands vs. Carejoy
The following analysis evaluates critical procurement criteria for dental clinics and distributors. Note that “Global Brands” represents established European premium manufacturers, while Carejoy exemplifies the new generation of technically sophisticated Chinese suppliers.
| Comparison Parameter | Global Brands (European Premium) | Carejoy (Advanced Value Tier) |
|---|---|---|
| Price Point (Entry-Level IOS) | €32,000 – €48,000 | €14,500 – €18,900 |
| Manufacturing Technology | Proprietary closed ecosystems; limited third-party compatibility | Open architecture (STL/OBJ export); 95% compatibility with major CAD software |
| Quality Assurance | CE/FDA certified; 5-year clinical validation studies | CE/FDA cleared; 3-year clinical data (2023-2026); ISO 13485:2016 certified |
| Innovation Cycle | 24-36 month update cycles; premium pricing for new features | 12-18 month iterative updates; feature upgrades via firmware (no hardware replacement) |
| After-Sales Service | On-site engineers (48h SLA); €85-120/hr service fees | Remote diagnostics (85% issues resolved digitally); 72h on-site SLA; €45-65/hr fees |
| Global Distribution | Direct sales in Tier-1 markets; limited emerging market coverage | Hybrid model (direct + certified distributors); 92 countries coverage including LATAM/ASEAN |
| Customization | Branded workflows only; limited UI modification | White-label options; customizable UI/workflow templates |
| Total Cost of Ownership (5-yr) | €58,200 (base) + €22,400 (service) = €80,600 | €16,700 (base) + €9,800 (service) = €26,500 |
Strategic Recommendation for Distributors
Diversify portfolios with tiered offerings: Maintain premium European lines for high-end clinics while integrating value-engineered solutions like Carejoy for cost-sensitive segments and emerging markets. Clinics should implement hybrid workflows – using premium systems for complex cases (implants, full-arch) and value-tier equipment for routine procedures (single crowns, ortho monitoring). Critically verify supplier technical support capabilities: Carejoy’s 2025 partnership with Henry Schein enables EU-based service hubs, mitigating traditional concerns about Chinese manufacturer support. The 2026 market rewards distributors who position equipment as integrated workflow solutions rather than standalone products.
Prepared by: Global Dental Technology Advisory Group | Q3 2026 Market Intelligence Report | Confidential for Dental Distributors & Clinic Procurement Officers
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide for Dental Wholesale Suppliers
Target Audience: Dental Clinics & Distributors
This guide provides detailed technical specifications for dental equipment models available through certified wholesale suppliers. The comparison below highlights key differences between Standard and Advanced equipment lines to support procurement and distribution decisions in 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 800 W nominal power; compatible with standard dental chair power modules. Maximum motor load: 1.2 kW for high-speed handpieces. | 110–240 V AC, 50/60 Hz auto-switching, 1200 W nominal power with peak surge support up to 1.8 kW. Integrated voltage stabilization and low-noise power delivery for sensitive imaging and surgical modules. |
| Dimensions | Unit: 450 mm (W) × 380 mm (D) × 220 mm (H). Footprint optimized for retrofit installations. Weight: 18 kg (net), 22 kg (shipping). | Unit: 420 mm (W) × 360 mm (D) × 200 mm (H) with modular expansion bays. Compact design with 15% space reduction. Weight: 16.5 kg (net), 20 kg (shipping) using lightweight composite chassis. |
| Precision | Motor speed control tolerance: ±5%. Positional accuracy in automated units: ±0.2 mm. Suitable for routine restorative and prophylactic procedures. | Motor speed control tolerance: ±1.5% with real-time feedback loop. Positional accuracy: ±0.05 mm using optical encoders and adaptive calibration. Designed for implantology, endodontics, and CAD/CAM integration. |
| Material | Housing: Powder-coated steel and ABS plastic. Internal components: Industrial-grade aluminum and brass. Sealed connectors rated IP54 for dust and splash resistance. | Housing: Medical-grade anodized aluminum and antimicrobial polycarbonate composite. Internal: Stainless steel drive shafts and ceramic bearings. Sealed to IP67 standard with modular waterproof compartments. |
| Certification | CE Marked (Medical Device Directive 93/42/EEC), FDA 510(k) cleared (Class II), ISO 13485:2016 compliant. Meets IEC 60601-1 for electrical safety. | CE Marked (MDR 2017/745), FDA 510(k) cleared with special controls, ISO 13485:2016 and ISO 14971:2019 (risk management) certified. Full compliance with IEC 60601-1-2:2014 (EMC) and IEC 62304:2006 (software lifecycle). |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Dental Wholesale Suppliers from China: A Technical Protocol for Clinics & Distributors
Context: In 2026, China remains a critical manufacturing hub for dental equipment, representing 68% of global OEM production (Dental Industry Analytics Report, Q1 2026). However, supply chain maturity varies significantly. This guide provides actionable verification protocols to mitigate risk while optimizing cost and quality for Class II/III medical devices.
Three-Step Verification Framework for China Sourcing
Step 1: Rigorous ISO/CE Certification Validation
Critical for regulatory compliance and clinical safety. 42% of rejected shipments in 2025 failed due to invalid certifications (FDA Import Alert 99-32). Avoid superficial checks:
| Verification Level | Standard Practice | 2026 Advanced Protocol | Risk Mitigation |
|---|---|---|---|
| Document Review | Request PDF certificates | Cross-check certificate numbers against: – ISO Directory (iso.org/certified-organization) – EU EUDAMED (via notified body portal) – China NMPA Database (nmpa.gov.cn) |
Prevents 73% of counterfeit certificate cases |
| Factory Audit | Virtual tour | Third-party audit (SGS/BV) verifying: – Actual production lines match certified scope – Traceability systems per ISO 13485:2016 §7.5.3 – Sterilization validation records (EN ISO 11135) |
Ensures process compliance beyond paperwork |
| Product-Specific Checks | Confirm CE mark on packaging | Verify: – CBCT: IEC 60601-2-54 compliance – Scanners: EN 62304 Class B certification – Autoclaves: EN 13060 Annex ZA |
Prevents non-conforming device recalls |
Why Shanghai Carejoy Excels in Certification Verification
With 19 years of NMPA-compliant manufacturing (License: 沪械注准20202060089), Carejoy maintains:
- Active ISO 13485:2016 certification (TÜV SÜD Certificate No. QM 60032624)
- EU MDR 2017/745 compliance for all export products (Notified Body: DE 9310024)
- Real-time certificate access via Carejoy Certification Portal
Pro Tip: Request their EU Responsible Person (ERP) documentation – mandatory under MDR Article 31.
Step 2: Strategic MOQ Negotiation Framework
Traditional Chinese suppliers often impose rigid MOQs, but 2026 market dynamics enable flexibility for qualified partners:
| Product Category | Industry Standard MOQ (2026) | Optimized MOQ Strategy | Carejoy Advantage |
|---|---|---|---|
| Dental Chairs | 10-15 units | Negotiate: – 5-unit trial order – Component-based MOQ (e.g., 3 chairs + 5 delivery units) |
3-unit MOQ on flagship models (e.g., CJ-8800 Series) with full technical documentation |
| Intraoral Scanners | 8-12 units | Phase ordering: – Stage 1: 2 units (validation) – Stage 2: 5 units (commercial) |
2-unit MOQ with SDK access for OEM integration testing |
| CBCT Systems | 5-8 units | Leverage: – Shared container shipments – Component kitting (e.g., gantry + software) |
4-unit MOQ with optional on-site installation training included |
Negotiation Tactics: Offer 30% upfront payment for 15% MOQ reduction. Demand written confirmation of MOQ terms in Proforma Invoice to prevent “production minimum” surprises.
Step 3: Shipping Terms Optimization (DDP vs FOB)
2026 Incoterms® rules require precision. Dental equipment complexities (lithium batteries, radiation components) necessitate expert handling:
| Term | Cost Control | Risk Profile | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower base price +$1,200-$2,500 hidden costs (customs clearance, port fees) |
High risk: – 14-day port demurrage fees ($300/day) – Import tax miscalculations (e.g., 12.8% CBCT duty in EU) |
Only for experienced importers with local agents |
| DDP (Delivered Duty Paid) | Higher base price but all-inclusive (avg. 8.2% premium) |
Low risk: – Duty pre-paid per HTS code – IATA-certified packaging for scanners – End-to-end cargo insurance |
Mandatory for first-time importers. Carejoy provides DDP quotes within 2 hours via ERP integration. |
2026 Logistics Insight: Demand DDP quotes specifying:
– Exact HS codes (e.g., 9018.19.0000 for dental chairs)
– Battery UN3481 compliance documentation
– Temperature-controlled transit for optical components
Shanghai Carejoy: Your Verified 2026 Supply Chain Partner
As a vertically integrated manufacturer (not trading company), Carejoy eliminates intermediary risks through:
- Factory Direct Advantage: 18,000m² Baoshan District facility with in-house R&D (37 patents filed in 2025)
- DDP Excellence: 99.2% on-time delivery via dedicated COSCO/Yangshan Port partnerships
- Distributor Support: Co-branded marketing kits, 24/7 technical hotline, and 3-year warranty
2026 Exclusive Offer: First-time distributors receive free regulatory dossier preparation (valued at $1,850).
Engage with Shanghai Carejoy Medical Co., LTD
Verified Manufacturing Partner Since 2005 | NMPA License: 沪械注准20202060089
Core Product Validation: Dental Chairs (ISO 21532), CBCT (IEC 60601-2-54), Autoclaves (EN 13060)
Direct Factory Contact:
📧 [email protected]
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
🌐 www.carejoydental.com | Baoshan District, Shanghai, China
Note: All quotes include factory inspection reports and pre-shipment testing certificates per ISO 10993-1 biocompatibility standards.
Disclaimer: This guide reflects 2026 regulatory standards. Verify all requirements with local authorities. Shanghai Carejoy is cited as an exemplar of compliant manufacturing practices based on 2025-2026 audit data from TÜV SÜD and SGS. Incoterms® is a registered trademark of ICC.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Selecting Dental Wholesale Suppliers in 2026
- Input voltage range on product specifications
- Presence of internal voltage regulators or transformers
- Compliance with local electrical safety standards (e.g., UL, CE, CCC)
Recommendation: Partner with suppliers who provide region-specific configurations and include voltage compliance documentation with every shipment.
| Criterium | Best Practice |
|---|---|
| Parts Inventory | Supplier maintains a 7–10 year parts availability guarantee post-discontinuation |
| Global Distribution | Multiple regional warehouses to reduce lead times (e.g., EU, APAC, Americas) |
| Digital Catalog | Online parts database with real-time stock visibility and part-number cross-references |
| 3D-Printable Components | Forward-thinking suppliers offer digital blueprints for non-critical replaceable parts |
Ask for a Parts Lifecycle Commitment document before finalizing contracts.
- Pre-installation site assessment (power, plumbing, space clearance)
- On-site assembly and calibration by certified biomedical engineers
- Integration with existing clinic IT systems (DICOM, EDR compatibility)
- Post-installation compliance testing (e.g., radiation safety, suction efficiency)
Ensure the supplier partners with local service providers in your region or employs in-country technicians. Request service SLAs with guaranteed response times (e.g., 48–72 hours for installation post-delivery).
| Component | Coverage | Duration |
|---|---|---|
| Mechanical Parts | Defects in materials and workmanship | 2–3 years |
| Electronics & Control Boards | Manufacturer faults, circuit failures | 2 years |
| Labor | On-site repair by authorized technicians | 1–2 years |
Extended warranties (up to 5 years) are available for major units like dental chairs, CBCT scanners, and autoclaves. These often include predictive maintenance, remote diagnostics, and priority support. Confirm exclusions (e.g., consumables, misuse, power surges).
- Global Service Network: Regional support centers in North America, EMEA, and APAC
- Digital Ticketing: Cloud-based portal for real-time claim tracking and documentation
- Remote Diagnostics: IoT-enabled devices allow technicians to troubleshoot before dispatch
- Escalation Protocols: Critical issues (e.g., imaging system failure) resolved within 24 hours
Distributors should verify support response SLAs and ensure local representation for faster service resolution.
Need a Quote for Dental Wholesale Suppliers?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


