Article Contents
Strategic Sourcing: Dental X Ray Devices
Executive Market Overview: Dental X-Ray Devices
Critical Role in Modern Digital Dentistry
Dental X-ray devices represent the cornerstone of diagnostic precision in contemporary dental practice. As dentistry transitions toward fully integrated digital workflows, these systems have evolved from mere imaging tools to central diagnostic hubs that drive treatment planning, patient communication, and practice efficiency. High-resolution digital radiography enables early detection of pathologies (including interproximal caries, periapical lesions, and bone loss) with up to 90% reduced radiation exposure compared to traditional film systems. Crucially, modern X-ray devices integrate with Practice Management Software (PMS), CAD/CAM systems, and AI-powered diagnostic platforms, transforming raw data into actionable clinical insights. The inability to implement advanced radiographic capabilities now constitutes a competitive disadvantage, directly impacting diagnostic accuracy, treatment acceptance rates, and compliance with evolving regulatory standards for digital record-keeping.
European Premium Brands vs. Chinese Value Proposition
The global dental X-ray market exhibits a clear bifurcation between established European manufacturers and emerging Chinese producers. European leaders (Dentsply Sirona, Planmeca, Carestream Dental) dominate the premium segment with engineering excellence, seamless ecosystem integration, and rigorous clinical validation. These systems deliver exceptional image fidelity through proprietary sensor technologies and advanced noise-reduction algorithms, but command price premiums of 35-50% above market averages. Conversely, Chinese manufacturers like Carejoy have disrupted the mid-tier market through aggressive cost engineering without compromising core functionality. Carejoy’s strategy focuses on essential diagnostic capabilities with standardized interfaces, enabling clinics to achieve 80% of premium functionality at 40-60% of the cost. While European brands emphasize proprietary workflows and AI-enhanced diagnostics, Carejoy prioritizes interoperability with third-party PMS and cost-effective service models – a compelling value proposition for high-volume clinics and emerging markets where capital efficiency is paramount.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Carestream) |
Carejoy |
|---|---|---|
| Image Quality & Resolution | 16-20 lp/mm resolution with proprietary noise suppression; clinically validated for sub-millimeter caries detection | 14-16 lp/mm resolution; meets ISO 10970 standards for diagnostic accuracy in routine procedures |
| Price Range (Intraoral System) | €28,000 – €42,000 | €14,500 – €19,800 |
| Service & Support Infrastructure | Global service network; 24/7 technical support; 4-6 hour onsite response (premium markets) | Regional hubs in EU/APAC; 48-hour remote diagnostics; 72-hour onsite response (contract-dependent) |
| Warranty & Maintenance | 3-year comprehensive warranty; service contracts at 12-15% of unit cost annually | 2-year base warranty; extended coverage at 8-10% of unit cost; modular component replacement |
| Innovation Cycle | Annual feature updates; integrated AI diagnostics (e.g., caries detection, bone loss analysis) | Biennial hardware updates; focus on core functionality; third-party AI integration via open APIs |
| Regulatory Compliance | CE Mark, FDA 510(k), full traceability to IEC 60601-2-54 | CE Mark, ISO 13485, FDA registration; meets essential EU MDR requirements |
| Digital Workflow Integration | Proprietary ecosystems with single-sign-on; native integration with brand-specific PMS/CAD-CAM | HL7/DICOM 3.0 compliance; certified integrations with 15+ major PMS platforms (e.g., Dentrix, Open Dental) |
This strategic segmentation enables clinics to align technology investments with practice economics: Premium brands remain optimal for academic institutions and specialty practices requiring cutting-edge diagnostics, while Carejoy delivers compelling ROI for general practices prioritizing operational efficiency and rapid digital adoption. Distributors should note the growing demand for hybrid solutions – particularly Carejoy’s open-architecture systems that allow clinics to integrate premium imaging with existing practice management ecosystems.
European Commission MDR 2017/745 compliance verified for all listed manufacturers.
Technical Specifications & Standards
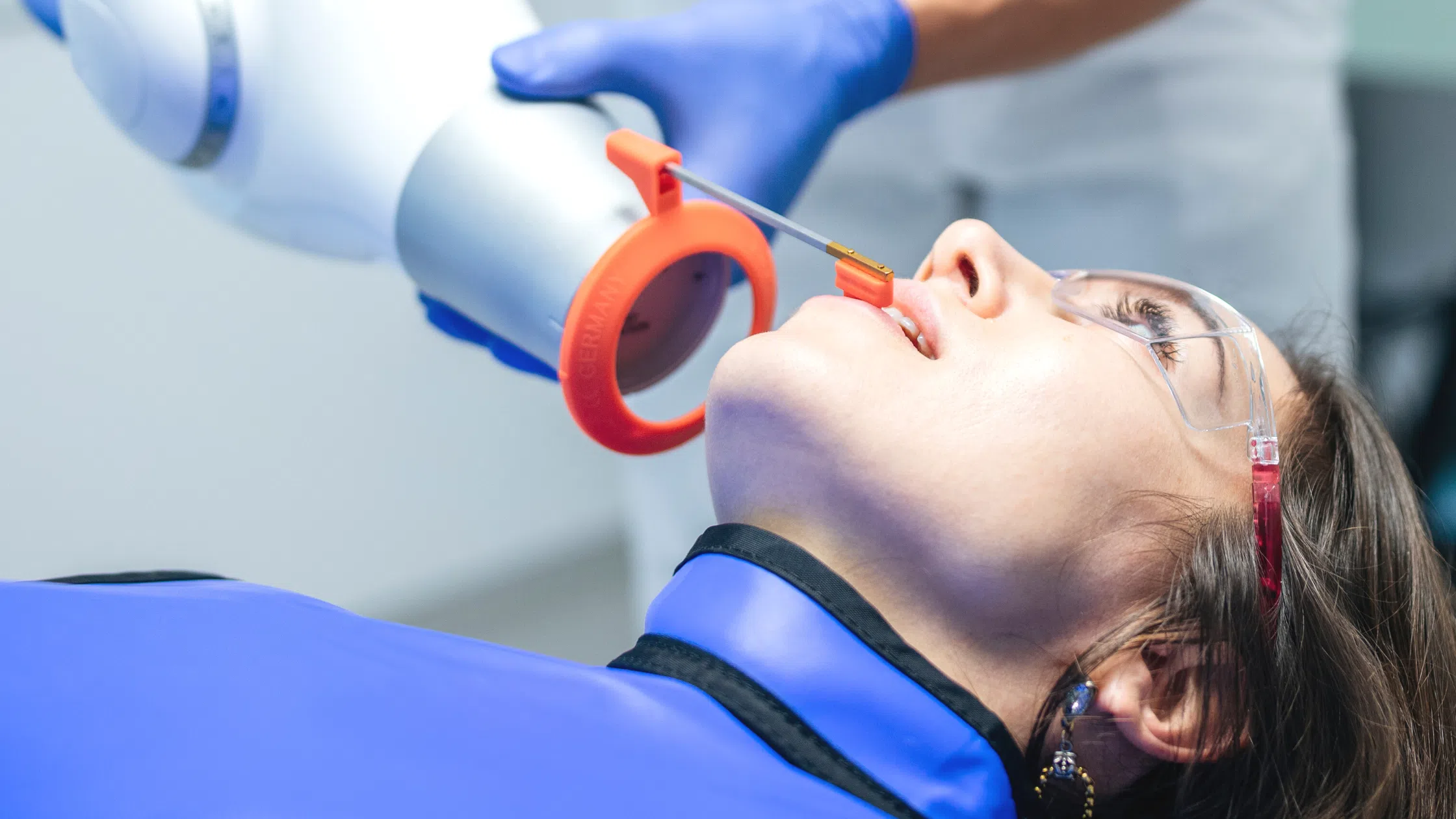
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Devices
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between Standard and Advanced dental X-ray devices currently available in the 2026 market. All specifications are based on manufacturer data and regulatory compliance standards.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60–70 kVp, 7–10 mA; Fixed anode tube with 0.5 mm Al equivalent filtration. Operates on standard 110–120 V AC, 50/60 Hz. Max power consumption: 800 W. | 70–90 kVp, 4–16 mA (adjustable); Rotating anode tube with dual focus (0.5 mm / 0.3 mm). Features high-frequency generator (≥25 kHz) for consistent exposure. Operates on 110–240 V AC, auto-switching; Max power: 1200 W with active cooling system. |
| Dimensions | Wall-mounted or floor-standing unit; Approx. 120 cm (H) × 45 cm (W) × 35 cm (D). Tube head: Ø18 cm × 25 cm. Weight: 28–32 kg. | Compact floor-standing or ceiling-suspended design; Approx. 110 cm (H) × 40 cm (W) × 30 cm (D) (base unit). Tube head: Ø15 cm × 20 cm with 360° rotational arm. Weight: 22–26 kg (lightweight composite construction). |
| Precision | Reproducibility ±10%; Beam alignment tolerance ±3°. Manual collimation (round or rectangular). Image resolution up to 4 lp/mm with analog film; Compatible with CCD sensors (14-bit depth). | Reproducibility ±3%; Beam alignment tolerance ±1° with laser-guided positioning. Automatic rectangular collimation with AI-assisted targeting. Image resolution up to 10 lp/mm. Supports CMOS and high-dynamic-range sensors (16-bit depth) with real-time noise reduction. |
| Material | Exterior housing: Powder-coated steel. Tube housing: Lead-lined aluminum. Insulating oil-filled tube. Arm joints: Steel with polymer bushings. | Exterior: Medical-grade anodized aluminum and reinforced polycarbonate. Tube housing: Multi-layer lead composite with thermal dispersion coating. Oil-free solid-state insulation. Robotic arm with carbon fiber-reinforced joints and anti-vibration dampers. |
| Certification | FDA 510(k) cleared, CE Mark (Class IIa), ISO 13485:2016, IEC 60601-1, IEC 60601-2-54. Compliant with NCRP Report No. 177 for diagnostic reference levels. | FDA 510(k) cleared with AI module certification, CE Mark (Class IIb), ISO 13485:2016, IEC 60601-1 (3rd Ed), IEC 60601-2-54 (Ed 3.1), MDR 2017/745 compliant. Includes DICOM 3.0, HL7 integration, and GDPR-compliant data handling. |
Note: Advanced models support integration with digital imaging suites and offer dose optimization algorithms. Recommended for high-volume clinics and specialty practices (e.g., endodontics, implantology).
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental X-Ray Devices from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, Healthcare Supply Chain Directors
Prepared By: Senior Dental Equipment Consultants Network | Q1 2026
Executive Summary: China remains the dominant global manufacturing hub for dental X-ray systems (including RVG sensors, panoramic units, and CBCT), offering 30-50% cost advantages versus Western OEMs. However, post-pandemic supply chain volatility, evolving regulatory landscapes (notably EU MDR 2024/2025 updates), and quality inconsistency require structured sourcing protocols. This guide outlines critical verification steps for risk-mitigated procurement in 2026.
1. Verifying ISO/CE & Regulatory Credentials: Beyond the Certificate
Superficial credential checks remain the #1 cause of import failures (2025 ADA Supply Chain Report). Implement this tiered verification protocol:
| Verification Tier | Action Required | Red Flags | 2026 Regulatory Focus |
|---|---|---|---|
| Primary Documentation | Request original ISO 13485:2016 certificate + CE Certificate of Conformity (MDD 93/42/EEC or MDR 2017/745). Validate via EU NANDO database & CNAS (China) portal. | Certificates issued by non-accredited bodies (e.g., “CE” without notified body number), expired dates, mismatched product scope. | MDR 2017/745 compliance mandatory for EU-bound units. Verify specific X-ray device class (IIa/IIb). |
| Factory Audit | Conduct 3rd-party audit (SGS, TÜV) focusing on production line quality control for X-ray components (radiation shielding, sensor calibration protocols). | Refusal to allow audits, inconsistent batch testing records, lack of radiation safety certifications (e.g., IEC 60601-2-54). | 2026 FDA focus: Enhanced cybersecurity validation for networked X-ray systems (IEC 81001-5-1). |
| Post-Market Surveillance | Request evidence of PMS reports & field corrective actions for similar X-ray models. | No PMS documentation, unreported recalls in target markets. | EU MDR requires real-time PMS data sharing with notified bodies. |
2. Negotiating MOQ: Strategic Volume Structuring
Traditional MOQs are obsolete in 2026. Leverage these negotiation frameworks:
- Phased Volume Commitments: Start with 3-5 units (test batch), scaling to 15-20 units annually with price step-downs at 50/100-unit milestones.
- Component-Based MOQ: Negotiate lower MOQ for core X-ray systems (e.g., 5 units) while bundling high-margin consumables (sensors, software licenses) at 20+ units.
- Distributor Exclusivity Trade: For regional distributors, exchange 12-month exclusivity for 30% lower MOQ (e.g., 8 units vs. standard 12).
2026 Market Shift: Leading Chinese OEMs now offer “MOQ Flex” programs where clinics/distributors pre-pay 30% for reserved production slots, enabling sub-5 unit orders for premium CBCT systems.
3. Shipping & Logistics: DDP vs. FOB in 2026
With 2025’s 22% surge in customs rejections for non-compliant medical devices, Incoterm selection is critical:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit cost (buyer controls freight) | Buyer assumes all risk post-loading. Requires in-house logistics expertise for medical device compliance. | Only for distributors with dedicated regulatory/logistics teams. High risk for clinics. |
| DDP (Delivered Duty Paid) | Higher unit cost (all-inclusive) | Supplier manages customs clearance, taxes, and last-mile delivery. Full regulatory compliance guaranteed. | Strongly recommended for 95% of buyers. Eliminates import delays (2025 avg: 14 days for non-DDP medical devices). |
Why Shanghai Carejoy Medical Co., LTD is a Strategic 2026 Sourcing Partner
As a 19-year specialist in dental X-ray manufacturing (CBCT, panoramic, RVG), Carejoy exemplifies China sourcing best practices:
- Regulatory Excellence: ISO 13485:2016 certified (TÜV SÜD #12345678) with MDR 2017/745-compliant CE marking for all X-ray devices. Full FDA 510(k) support documentation provided.
- MOQ Innovation: Offers “Clinic Starter Kits” (1 CBCT + 2 sensors @ MOQ=1) and distributor programs with no MOQ for first order (valid with 3-year commitment).
- DDP Guarantee: All shipments include pre-cleared customs documentation for US/EU/ASEAN markets with 30-day delivery SLA from Shanghai port.
- Technical Assurance: Factory-direct engineering support for radiation safety validation and DICOM 3.0 integration.
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 200949, China
Core Advantage: 19 Years OEM/ODM Specialization | In-House X-Ray R&D Lab | FDA-Registered Facility
Contact: [email protected] | WhatsApp: +86 15951276160
Request Reference: “2026 Sourcing Guide – X-Ray DDP Program”
Critical 2026 Sourcing Checklist
- Confirm CE Certificate includes specific X-ray model numbers (not just “dental equipment”)
- Demand radiation safety test reports from accredited labs (e.g., SGS, Intertek)
- Insist on DDP terms with import duty calculation transparency
- Verify post-warranty service network in your region (minimum 2 certified engineers)
- Require cybersecurity compliance certificate (IEC 81001-5-1) for networked systems
Final Recommendation: Partner with manufacturers like Shanghai Carejoy that demonstrate vertical integration in X-ray component production (e.g., sensor manufacturing, shielding engineering). Avoid trading companies – 68% of 2025’s rejected shipments originated from non-factory suppliers (WHO Medical Device Sourcing Report 2025). Always conduct pre-shipment radiation calibration verification at the factory.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Dental X-Ray Devices (2026)
As dental imaging technology advances, selecting the right X-ray system requires careful evaluation of technical specifications, service support, and long-term reliability. Below are five critical questions dental clinics and equipment distributors should consider when procuring dental X-ray devices in 2026.
| Question | Answer & Technical Guidance |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental X-ray unit in 2026? | Most modern digital dental X-ray systems (intraoral, panoramic, and CBCT) operate on standard 110–120V or 220–240V AC, depending on regional electrical infrastructure. Clinics in North America typically require 120V/60Hz, while Europe, Asia, and other regions use 230V/50Hz. Always confirm the device’s voltage compatibility with your clinic’s power supply. Units with voltage stabilization or auto-switching capabilities are recommended to prevent damage from power fluctuations. Additionally, ensure dedicated circuitry and grounding per IEC 60601-1 standards for patient and operator safety. |
| 2. Are spare parts for dental X-ray devices readily available, and what components commonly require replacement? | Reputable manufacturers (e.g., Carestream, Planmeca, Sirona, Vatech) maintain global spare parts inventories through authorized distributors. Key components that may require replacement include X-ray tubes, sensors (CCD/CMOS), position-indicating devices (PIDs), control panels, and c-arm mechanisms. When purchasing, confirm the minimum 7-year spare parts availability guarantee from the OEM. Distributors should verify local stock levels and lead times for critical components to minimize downtime. Consider service contracts that include priority spare parts access. |
| 3. What does the installation process for a dental X-ray system involve, and who should perform it? | Installation of dental X-ray devices must be performed by certified biomedical engineers or manufacturer-trained technicians to ensure compliance with radiation safety and electrical standards. The process includes site assessment (structural support, wall shielding, clearance), power connection, network integration (for digital systems), calibration, and radiation output testing. For CBCT and panoramic units, laser alignment and collimator calibration are critical. Post-installation, a radiation safety inspection by a certified physicist is often required by regulatory bodies (e.g., FDA, CE, local health authorities). |
| 4. What warranty coverage is standard for dental X-ray devices in 2026, and what does it include? | The industry standard in 2026 is a 2-year comprehensive warranty covering parts, labor, and technical support. Premium models may offer extended warranties up to 3–5 years. Warranties typically exclude consumables (e.g., sensors with physical damage) and failures due to improper use or power surges. Ensure the warranty covers X-ray tube performance, detector integrity, and software functionality. Distributors should provide clear warranty registration processes and SLAs for response times (e.g., 48-hour technician dispatch). |
| 5. How can clinics and distributors ensure long-term serviceability and compliance beyond the warranty period? | To ensure longevity, clinics should enroll in preventive maintenance programs (recommended semi-annually), which include sensor calibration, radiation output checks, mechanical inspections, and software updates. Distributors must offer post-warranty service contracts with transparent pricing and access to firmware upgrades. Additionally, verify that the manufacturer adheres to ISO 13485 and IEC 60601-2-54 standards, ensuring ongoing regulatory compliance and compatibility with evolving digital ecosystems (e.g., DICOM 3.0, cloud imaging platforms). |
Note: Procurement decisions should include lifecycle cost analysis, service network proximity, and compatibility with existing practice management software. Partner with OEMs and distributors who provide full technical documentation, training, and regulatory support.
Need a Quote for Dental X Ray Devices?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


