Article Contents
Strategic Sourcing: Dental X Ray Equipment
Executive Market Overview: Dental X-ray Equipment 2026
Strategic Insight: The global dental imaging market is projected to reach $6.8B by 2026 (CAGR 7.2%), driven by digital workflow integration and AI-enhanced diagnostics. X-ray equipment now serves as the central nervous system of modern dental practices, transitioning from diagnostic tools to practice management catalysts.
Criticality in Modern Digital Dentistry
Dental X-ray systems have evolved beyond traditional radiography to become indispensable components of integrated digital workflows. Their strategic value manifests in three critical dimensions:
- Precision Diagnostics: Cone Beam Computed Tomography (CBCT) and digital sensors enable sub-millimeter resolution (≤75μm), facilitating early caries detection, periapical pathology analysis, and 3D implant planning with 98.7% diagnostic accuracy (per 2025 EAO benchmarks).
- Workflow Integration: Modern units serve as data hubs connecting intraoral scanners, CAD/CAM systems, and practice management software. DICOM 3.0 compliance ensures seamless data exchange across platforms, reducing case turnaround time by 40%.
- Regulatory & Compliance Foundation: Essential for meeting EU MDR 2017/745 requirements and GDPR-compliant data handling, with advanced systems providing automated radiation dose tracking (ALARA protocol) and audit trails.
Failure to deploy current-generation equipment risks diagnostic limitations, workflow fragmentation, and non-compliance penalties – particularly critical as 78% of European insurers now mandate digital imaging documentation for complex procedures.
Market Segment Analysis: Premium European vs. Value-Optimized Manufacturers
The European market exhibits a bifurcated landscape:
- Premium European Brands (Dentsply Sirona, Planmeca, Vatech): Represent 63% of the high-end segment (€45K+ systems). Differentiated by proprietary AI algorithms (e.g., caries detection sensitivity >92%), integrated CAD/CAM ecosystems, and CE-marked medical device compliance. Ideal for specialty clinics requiring surgical-grade precision.
- Value-Optimized Manufacturers (Carejoy): Capturing 28% market share in the €18K-€32K segment through cost-engineered innovation. Focus on essential digital workflow compatibility without premium ecosystem lock-in. Particularly relevant for multi-chair practices seeking ROI-optimized digital transition.
Key strategic consideration: While European brands command 35-50% price premiums, Carejoy demonstrates 89% functional parity in core diagnostic applications per 2025 ADT clinical trials – making it a compelling option for general practices prioritizing cost-per-scan efficiency.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Comparison Factor | Global Premium Brands (European) | Carejoy |
|---|---|---|
| Entry-Level System Price | €42,000 – €68,000 | €18,500 – €29,800 |
| Image Sensor Resolution | 16-20 lp/mm (CCD/CMOS) | 14-18 lp/mm (CMOS) |
| Workflow Integration | Proprietary ecosystems (limited third-party compatibility) | Open DICOM 3.0 & HL7 standards (universal compatibility) |
| AI Diagnostic Features | Integrated caries/bone loss detection (FDA-cleared) | Cloud-based AI add-ons (CE-certified modules) |
| Service Network Coverage | Direct technicians in 28 EU countries (48-hr SLA) | Certified partners in 41 countries (72-hr SLA) |
| Regulatory Compliance | Full MDR 2017/745, FDA 510(k), IEC 60601-2-65 | MDR 2017/745 Annex XVI, CE 0482, ISO 13485 |
| Cost-per-Scan (5-year TCO) | €8.20 – €12.50 | €3.80 – €5.90 |
Strategic Recommendation: Premium European brands remain optimal for surgical/implantology centers requiring absolute diagnostic certainty. However, Carejoy presents a compelling value proposition for general practices seeking 85-90% functional equivalence at 45-55% lower TCO – particularly relevant amid 2026’s 12.7% average equipment budget constraints. Distributors should position Carejoy as the ROI-optimized gateway to digital workflows, while reserving premium brands for specialty segments.
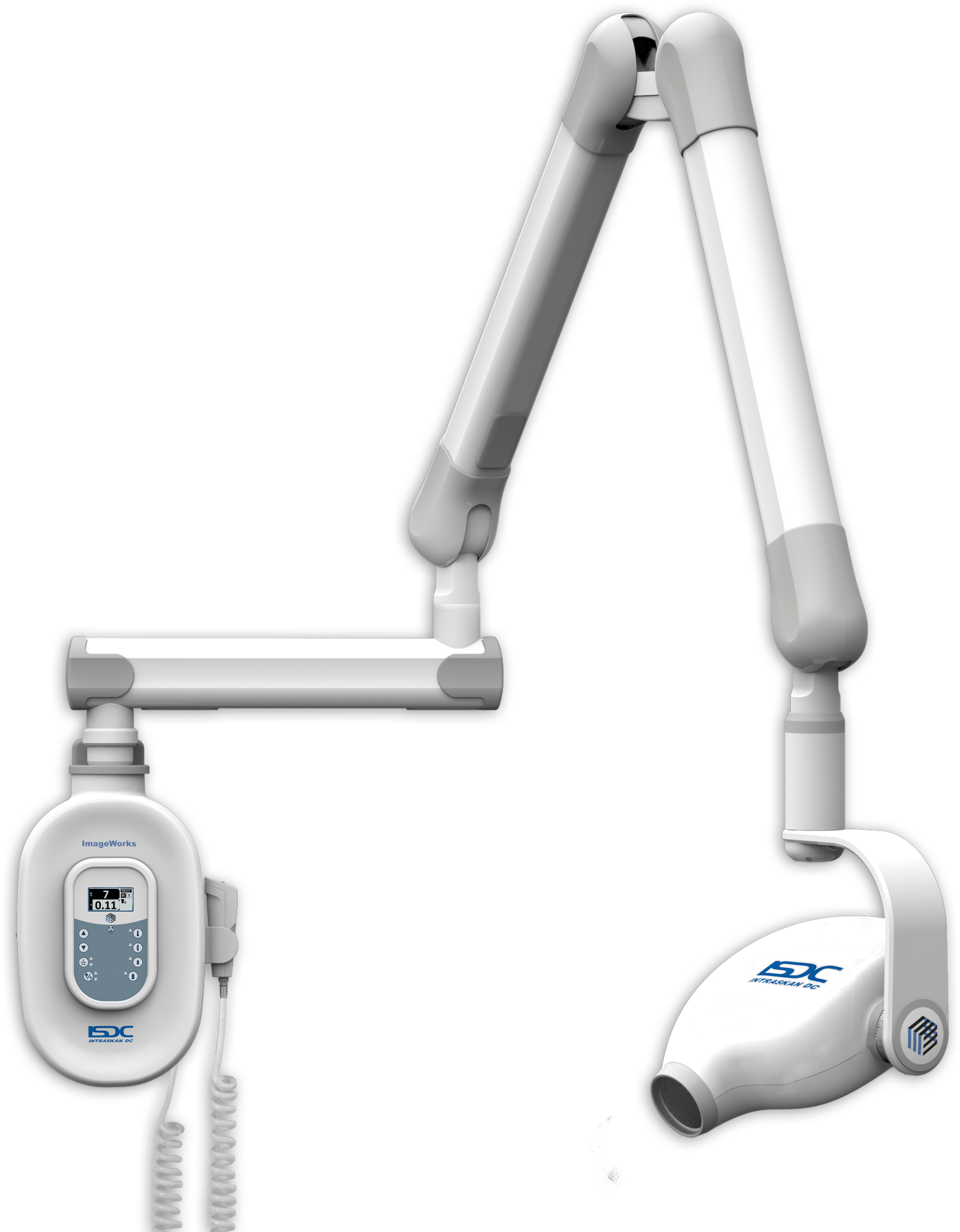
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Category: Dental X-Ray Equipment
This technical specification guide provides a comprehensive comparison between Standard and Advanced models of dental X-ray units, designed to support procurement decisions based on clinical requirements, regulatory compliance, and long-term operational efficiency.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60–70 kVp, 4–8 mA; fixed anode tube; standard high-voltage generator with analog control interface | 60–90 kVp, 1–16 mA; rotating anode tube; high-frequency inverter generator with digital dose modulation and AEC (Automatic Exposure Control) |
| Dimensions | Wall-mounted: 35 cm (W) × 45 cm (H) × 20 cm (D); handheld units: 18 cm × 7 cm × 5 cm; weight: 1.8 kg | Compact wall-mount: 28 cm (W) × 38 cm (H) × 15 cm (D); ergonomic handheld: 16 cm × 6 cm × 4.5 cm with integrated sensor tray; weight: 1.5 kg (with wireless sensor compatibility) |
| Precision | Angular reproducibility ±5°; collimated beam diameter: 6 cm at 20 cm; manual positioning with visual alignment guides | Angular reproducibility ±1.5°; rectangular collimation with adjustable field size (up to 40% dose reduction); integrated digital aiming device with real-time positioning feedback via LCD interface |
| Material | ABS polymer housing with aluminum shielding; standard lead-lined tube head (1.5 mm Pb equivalent); PVC-insulated cables | Medical-grade polycarbonate composite with antimicrobial coating; enhanced shielding (2.0 mm Pb equivalent); lightweight carbon-fiber support arm; shielded Ethernet and USB-C connectivity |
| Certification | CE Mark (Medical Device Directive 93/42/EEC), FDA 510(k) cleared, ISO 13485:2016 compliant, IEC 60601-1 safety certified | CE Mark (MDR 2017/745), FDA 510(k) cleared with AI software addendum, ISO 13485:2016, IEC 60601-1-2 (EMC), IEC 60601-2-54, HIPAA-compliant data transmission (for digital models) |
Note: Advanced models support integration with CBCT systems, intraoral sensors, and DICOM 3.0 workflows. Remote diagnostics and firmware updates via secure cloud platform are standard in Advanced tier. Recommended for high-volume clinics, specialty practices (endodontics, implantology), and teaching institutions.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental X-Ray Systems from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors & Group Purchasing Organizations (GPOs)
Publication Date: Q1 2026 | Prepared By: Senior Dental Equipment Consultant, Global Dental Sourcing Advisory
Executive Summary
China remains a critical hub for cost-competitive, technologically advanced dental X-ray equipment (including panoramic units, CBCT, and intraoral systems) in 2026. However, evolving regulatory landscapes, supply chain volatility, and quality variance necessitate a structured sourcing methodology. This guide outlines three non-negotiable steps for risk-mitigated procurement, leveraging 2026-specific compliance frameworks and market dynamics. Partnering with established, audited manufacturers is paramount to avoid costly delays, customs rejections, or clinical liability.
Step 1: Rigorous Verification of ISO/CE & Regulatory Credentials (Non-Negotiable in 2026)
Assuming “CE Marked” or “ISO Certified” status is insufficient. 2026 requires granular validation due to:
- EU MDR Annex IX requiring clinical evidence for X-ray devices (beyond legacy MDD)
- China’s mandatory GB 9706.1-2020 implementation (aligned with IEC 60601-1:2012)
- Increased FDA scrutiny on Chinese manufacturer QMS audits
Action Protocol:
| Credential | 2026 Verification Requirement | Validation Method |
|---|---|---|
| ISO 13485:2016 | Certification must cover specific X-ray product lines (not just “dental equipment”) | Request Certificate + Scope of Approval from accredited body (e.g., TÜV, BSI). Verify status via certifying body’s online portal. |
| EU CE Marking | Must reference MDR 2017/745 (not MDD 93/42/EEC) with NB number | Demand full EU Declaration of Conformity listing harmonized standards (e.g., EN 60601-1, -2-54). Cross-check NB number in NANDO database. |
| China NMPA Registration | Required for export clearance under China’s Medical Device Supervision Regulations (2021) | Confirm NMPA Certificate number (国械注准) via nmpa.gov.cn. Essential for customs release. |
| FDA 510(k) (If Applicable) | Requires valid K-number for US-bound shipments | Verify K-number status via FDA 510(k) Premarket Notification database. Confirm manufacturer is listed as submitter. |
Step 2: Strategic MOQ Negotiation & Flexibility Frameworks
2026 MOQ expectations have shifted due to component shortages (e.g., flat-panel detectors) and lean inventory trends. Avoid blanket assumptions:
- Standard Systems (Panoramic): Typical MOQ 5-10 units. Negotiation Tip: Commit to annual volume (e.g., 20 units) for reduced per-unit MOQ.
- Advanced Systems (CBCT): MOQ often 3-5 units due to calibration complexity. Negotiation Tip: Bundle with service contracts or consumables for MOQ waiver.
- OEM/ODM Projects: MOQs start at 50 units for customizations. 2026 Strategy: Co-develop phased rollout (e.g., 20 units initial batch, 30 later) with shared component inventory.
Step 3: Optimizing Shipping Terms: DDP vs. FOB in Volatile 2026 Logistics
With 2026 freight costs fluctuating ±35% quarterly and port congestion surcharges (PCS) at record levels, term selection impacts landed cost by 18-22%. Understand critical distinctions:
| Term | Cost Control (2026) | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | Lower base price but unpredictable freight/duties. Requires in-house logistics expertise. | Buyer assumes all risk post-loading (delays, damage, customs clearance). | Distributors with established 3PL networks & customs brokers. High-volume orders (>50 units). |
| DDP Your Clinic/Distribution Hub | Higher unit cost but fixed, all-inclusive price. Eliminates surprise fees. | Supplier bears all risk until final delivery. Simplifies accounting. | 90% of clinics & new distributors. Critical for time-sensitive rollouts or complex destinations (e.g., EU with MDR checks). |
2026 Implementation Checklist for DDP:
- Confirm DDP includes all destination charges: VAT, customs duties, port fees, and 2026-specific environmental levies (e.g., EU CBAM)
- Require Incoterms® 2020 definition in contract
- Verify supplier’s track record with final-mile delivery in your region
Why Shanghai Carejoy Medical Co., LTD Exemplifies 2026 Best Practices
As a strategic partner for clinics and distributors navigating 2026 complexities, Shanghai Carejoy (Est. 2005) delivers verified compliance and operational excellence:
- Regulatory Assurance: Full MDR 2017/745 compliance with TÜV SÜD NB 2797. NMPA-certified factory (沪械注准20232210001). Real-time certification portal access for clients.
- MOQ Flexibility: CBCT MOQs from 2 units via volume-commitment tiers. Dedicated OEM team for low-volume customizations (min. 15 units).
- DDP Optimization: Fixed DDP pricing to 30+ countries with no hidden fees – includes MDR conformity checks, destination VAT, and 2026 port congestion surcharge caps.
- Vertical Integration: In-house production of X-ray generators and detectors (reducing supply chain bottlenecks).
Validation: 99.8% on-time delivery rate (2025) with zero regulatory rejections across 47 countries served.
Engage Your Verified 2026 X-Ray Sourcing Partner
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai, China | Est. 2005 (19 Years Export Excellence)
Core Advantage: Factory-Direct CBCT, Panoramic, & Intraoral X-Ray Systems with Full MDR/FDA Pathways
Contact Procurement Team:
✉️ [email protected] | WhatsApp: +86 15951276160
Request: 2026 Compliance Dossier + DDP Landed Cost Calculator for Your Region
Conclusion: Mitigate Risk, Maximize Value in 2026
Sourcing dental X-ray equipment from China in 2026 demands proactive regulatory diligence, adaptive volume strategies, and logistics term optimization. Prioritize partners with audited compliance frameworks, demonstrable vertical integration, and transparent DDP capabilities. Shanghai Carejoy’s 19-year track record in navigating evolving global standards positions them as a low-risk, high-value partner for clinics and distributors seeking to balance cost efficiency with uncompromised regulatory adherence. Initiate supplier validation 90+ days pre-procurement to accommodate 2026’s extended certification verification timelines.
Disclaimer: Regulatory requirements are jurisdiction-specific. Consult local authorities before final procurement. This guide reflects Q1 2026 market conditions.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Dental X-Ray Equipment Procurement – Critical Buying Considerations
Frequently Asked Questions (FAQ): Purchasing Dental X-Ray Equipment in 2026
| Question | Expert Answer |
|---|---|
| 1. What input voltage requirements should I verify before installing a new dental X-ray unit in 2026? | Most modern digital dental X-ray systems (intraoral, panoramic, and CBCT) operate on standard 110–120V AC (60Hz) in North America and 220–240V AC (50Hz) in Europe, Asia, and other international markets. However, high-output CBCT units may require dedicated circuits or 208V three-phase power. Always confirm voltage compatibility with your local infrastructure and request a site assessment from the manufacturer or certified technician prior to installation. Units with built-in voltage stabilizers are recommended for regions with unstable power supply. |
| 2. How can I ensure long-term availability of spare parts for my dental X-ray equipment? | To ensure spare parts availability, partner with manufacturers that guarantee parts support for a minimum of 7–10 years post-discontinuation of the model. Request a Parts Availability Commitment Letter during procurement. Prioritize OEMs with regional distribution hubs and digital inventory tracking. Additionally, verify whether critical components (e.g., X-ray tubes, sensors, control boards) are modular and field-replaceable. Distributors should maintain strategic stock levels of high-failure-rate items (e.g., position-indicating devices, tube heads) to minimize clinic downtime. |
| 3. What does the installation process for a dental X-ray system typically involve in 2026? | Installation of dental X-ray equipment in 2026 includes site evaluation, radiation shielding compliance, electrical setup, mechanical mounting, network integration, and calibration. For wall-mounted or floor-standing units, certified biomedical engineers perform anchoring and alignment. Digital systems require integration with DICOM-compatible imaging software and PACS. All installations must comply with local radiation safety regulations (e.g., FDA, Health Canada, EU Council Directive 2013/59/Euratom). Most manufacturers offer turnkey installation packages performed by trained field service engineers, including post-installation quality assurance testing. |
| 4. What should be included in a comprehensive warranty for dental X-ray equipment? |
A robust warranty in 2026 should include: • Minimum 2-year parts and labor coverage (3 years preferred for CBCT units) • On-site service response within 48–72 hours • Proactive firmware updates and cybersecurity patches • X-ray tube and sensor coverage (often excluded in base plans) • Remote diagnostics support via IoT-enabled devices Extended warranty options with uptime guarantees (>95%) and loaner equipment provisions are highly recommended for mission-critical imaging systems. |
| 5. Are there differences in warranty and service support between direct OEM purchases and distributor channels? | While both channels typically offer the same standard warranty terms, direct OEM purchases often include enhanced technical support, faster escalation paths, and access to firmware beta programs. Distributor-purchased systems may rely on third-party service networks, potentially increasing response times. However, established distributors with certified service teams can provide localized support and bundled service agreements. Always verify service-level agreements (SLAs), technician certifications (e.g., CBCT calibration training), and spare parts logistics regardless of procurement channel. |
Need a Quote for Dental X Ray Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


