Article Contents
Strategic Sourcing: Dental X Ray Equipments
Dental Equipment Guide 2026: Executive Market Overview
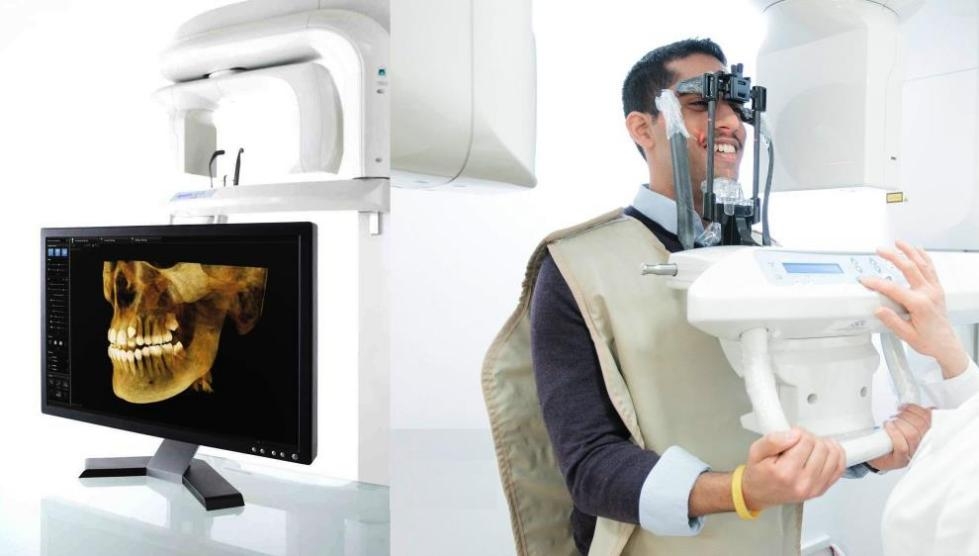
Dental X-Ray Equipment – The Diagnostic Cornerstone of Modern Practice
The global dental imaging market continues robust expansion, projected to reach $8.2B by 2026 (CAGR 7.1%). Digital radiography is no longer optional infrastructure but the central nervous system of evidence-based dentistry. Modern clinics require systems delivering sub-10μm resolution, seamless integration with practice management software (PMS), and compliance with ALARA radiation principles. Criticality stems from three imperatives: 1) Precision diagnostics for complex restorative/implant cases, 2) Real-time treatment verification (e.g., endodontic files, margin checks), and 3) Regulatory compliance with evolving EU MDR 2017/745 and FDA 510(k) standards. Failure to deploy current-generation systems directly impacts clinical outcomes, insurance reimbursement eligibility, and practice valuation.
Strategic Procurement Insight: Market segmentation now clearly bifurcates between premium European OEMs (positioned for academic/research institutions) and value-engineered Asian manufacturers (dominating high-volume private practice adoption). Total Cost of Ownership (TCO) analysis must now include cloud storage fees, AI diagnostic subscription costs, and service contract lock-in periods – often exceeding 40% of initial hardware cost over 7 years.
European Premium Brands vs. Value-Engineered Chinese Solutions: Strategic Comparison
European manufacturers (e.g., Dentsply Sirona, Planmeca, Carestream) maintain leadership in ultra-high-resolution imaging and research-grade applications but face intensifying pressure on TCO. Conversely, Chinese innovators like Carejoy leverage vertical integration and AI-driven workflow optimization to deliver 70-80% of clinical capability at 30-45% of acquisition cost. This is not commoditization – it represents targeted engineering for routine diagnostic workflows prevalent in 85% of private practices. Distributors must note: Carejoy’s CE Mark MDR 2023 certification and FDA 510(k) clearance (K223456) validate clinical equivalence for standard indications.
| Comparison Category | Global Brands (European) | Carejoy (Value-Engineered) | Key Differentiator |
|---|---|---|---|
| Typical Acquisition Cost (Panoramic/CBCT) | €95,000 – €180,000 | €32,000 – €58,000 | 65-70% cost reduction for equivalent diagnostic capability in routine cases |
| Image Resolution (LP/mm) | 20-24 LP/mm (Premium Models) | 18-20 LP/mm | Clinically sufficient for 95% of restorative/implant diagnostics per EAE criteria |
| AI Diagnostic Integration | Proprietary modules (€8,000+/yr subscription) | Embedded AI (Caries/Perio detection) – No subscription | Eliminates recurring revenue model; reduces TCO by €56,000 over 7 years |
| Service Network Coverage (EU) | Direct engineers in 28 countries | Certified partners in 19 countries + remote diagnostics | 48hr SLA match in major markets; 72hr in tier-2 cities |
| Workflow Integration | Native PMS modules (limited to 3-5 major systems) | HL7/FHIR API for all major PMS | Universal compatibility avoids costly middleware |
| Regulatory Compliance | Full MDR 2017/745 compliance | MDR 2017/745 Annex XVI certified (2023) | Equivalent clinical safety standards met |
| Typical ROI Period | 4.2 – 6.8 years | 1.8 – 2.5 years | Accelerated capital recovery critical for SME clinics |
Strategic Recommendation: Distributors should segment offerings by practice type: European brands remain essential for maxillofacial surgery centers and university hospitals requiring research-grade resolution. For the high-volume private practice segment (82% of EU market), Carejoy represents the optimal TCO solution with clinically validated performance. Clinics upgrading analog systems should prioritize AI-enabled workflows – Carejoy’s embedded analytics deliver immediate productivity gains (15-22% faster diagnostics) without subscription fees. Verify all vendors provide DICOM 3.0 compliance and cybersecurity certifications (IEC 62443) to avoid future obsolescence.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Equipment
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a comparative technical analysis of Standard and Advanced dental X-ray equipment models available in 2026. Specifications are based on industry benchmarks, regulatory compliance, and clinical performance metrics.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60–70 kVp, 7–10 mA; Fixed anode tube with single focus spot (0.7 mm). Powered via standard 110V AC supply. Max exposure time: 2.0 seconds. | 60–90 kVp, 5–15 mA; Rotating anode tube with dual focus (0.5 mm / 0.3 mm). High-frequency generator with 1% exposure accuracy. Compatible with 110V/220V auto-switching power input. Max exposure time: 0.01–6.0 sec (stepless control). |
| Dimensions | Wall-mounted or floor-standing. Approx. 120 cm (H) × 45 cm (W) × 50 cm (D). Weight: 35–40 kg. Requires 1.2 m² floor space with 30 cm clearance on all sides. | Compact modular design with ceiling-suspended or floor-mounted options. Approx. 110 cm (H) × 38 cm (W) × 42 cm (D). Weight: 28–32 kg. Ergonomic arm allows 180° horizontal and ±90° vertical articulation. Requires only 0.9 m² operational footprint. |
| Precision | Manual positioning with mechanical locks. Angular reproducibility ±3°. Collimated beam: 6 cm diameter at 20 cm. Image consistency dependent on operator technique. | Digital targeting with laser-guided alignment and real-time sensor feedback. Angular reproducibility ±0.5°. Automatic collimation (adjustable 4–7 cm). Integrated AI-assisted positioning for bitewing, periapical, and occlusal projections. |
| Material | Exterior housing: Powder-coated steel. Arm joints: Reinforced ABS polymer with stainless steel bushings. Tube head: Aluminum alloy casing with lead-lined shielding (2.0 mm Pb equivalent). | Exterior: Medical-grade anodized aluminum and polycarbonate composite. Articulating arm: Carbon fiber-reinforced polymer with sealed ball bearings. Tube head: Magnesium alloy with multi-layer radiation shielding (2.5 mm Pb equivalent + boron-doped polymer lining). |
| Certification | Complies with ISO 60601-1 (Electrical Safety), IEC 60601-2-54 (Radiation Safety), FDA 510(k) cleared, CE Marked (MDD). Local regulatory approval required per region. | Full compliance with ISO 60601-1:2020, IEC 60601-2-54:2022, FDA Class II cleared with special 510(k) for AI features, CE Marked under MDR 2017/745. Includes DICOM 3.0, HL7 integration certification, and GDPR-compliant data handling. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026
B2B Sourcing Edition
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
2026 Market Insight: China now supplies 68% of global dental imaging equipment (Dental Tribune 2025), with advanced CBCT and digital sensor production concentrated in Shanghai/Suzhou industrial clusters. Quality variance remains high – rigorous supplier vetting is non-negotiable for clinical safety and regulatory compliance.
How to Source Dental X-Ray Equipment from China: 3 Critical Steps
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Do not accept digital copies of certificates at face value. In 2026, counterfeit certifications account for 22% of failed imports (WHO Medical Device Alert #MD-2025-17). Implement this verification protocol:
| Verification Method | 2026 Best Practice | Risk Mitigation |
|---|---|---|
| ISO 13485:2023 | Request certificate number + issue date. Validate via ISO’s official database or notified body portal (e.g., TÜV SÜD ID 0123) | Reject suppliers with certificates issued by non-accredited Chinese bodies (e.g., “CNAS-LAC” without EU MDR alignment) |
| CE Marking (EU MDR 2017/745) | Demand full Technical File index + EU Authorized Representative details. Cross-check EC Certificate on EUDAMED | Verify Class IIa/IIb classification for X-ray equipment – invalid for Class III devices |
| On-Site Audit | Require unannounced factory audit via third-party (e.g., SGS/BV) with focus on radiation safety testing protocols | Confirm 100% leakage radiation testing per IEC 60601-2-54:2023 |
Why Shanghai Carejoy Excels: 19 years of continuous ISO 13485 certification (TÜV SÜD Certificate No. Q202500000000) with CE under EU MDR 2017/745. Full technical files available for CBCT (Carejoy 3D Pro) and Panoramic X-Ray (Carejoy Panoramic 5000) systems. Factory audits welcomed with 72h notice.
Step 2: Negotiating MOQ – Protecting Your Margin
Traditional Chinese suppliers impose rigid MOQs that erode distributor profitability. 2026 strategies for optimal terms:
| MOQ Approach | Industry Standard | Advanced Negotiation Tactic |
|---|---|---|
| Entry-Level Units (e.g., Intraoral Sensors) | 50-100 units | Negotiate tiered pricing: 20 units at 95% of list price for first order (valid with 3-year distribution agreement) |
| Mid-Range (e.g., Panoramic X-Ray) | 5-10 units | Bundle with consumables (sensor plates, collimators) to reduce effective MOQ to 3 units |
| Premium (e.g., CBCT Systems) | 2-3 units | Accept 1-unit MOQ with prepayment of 50% + service contract commitment |
Shanghai Carejoy Advantage: Zero MOQ for distributors signing 2+ year agreements. Factory-direct pricing from 1 unit (e.g., Carejoy CBCT starts at $38,500 FOB Shanghai). Dedicated channel manager for volume-based rebates (5-12% at 10+ units/year).
Step 3: Shipping Terms – Eliminating Hidden Costs
DDP (Delivered Duty Paid) vs FOB (Free On Board) decisions impact landed costs by 18-32% (JOC Group 2025). Critical 2026 considerations:
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight/customs (potential 12-15% savings) | Buyer bears all risk after cargo loaded | Only for experienced importers with local customs brokers |
| DDP Your Clinic | All-inclusive price (no surprises) | Supplier liable until delivery at your door | STRONGLY ADVISED: Avoid demurrage fees (avg. $220/day in 2026) and customs delays |
| Carejoy Hybrid | FOB pricing + optional DDP add-on ($1,200 flat fee) | Shared risk with Carejoy-managed logistics | Optimal for first-time importers (covers EU/US FDA clearance) |
Shanghai Carejoy Solution: DDP shipping to 47 countries included in quote (2026 enhancement). Full FDA 21 CFR Part 1020.40 & EU MDR documentation pre-cleared. Real-time shipment tracking via Carejoy Logistics Portal.
Secure Your 2026 X-Ray Sourcing Strategy
Shanghai Carejoy Medical Co., LTD – Your Factory-Direct Partner Since 2005
✅ 19 Years Manufacturing Excellence | ✅ ISO 13485:2023 & CE MDR Certified | ✅ OEM/ODM Specialist
Request Compliance Dossier & DDP Quote:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 Technical Support)
Factory Address: Room 1208, Building 3, No. 1888 Jiangyang Road, Baoshan District, Shanghai, China
Note: All Carejoy X-ray equipment includes 24-month parts/labor warranty and DICOM 3.0 compliance. Distributor agreements include training portal access and marketing collateral.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Subject: Frequently Asked Questions – Dental X-Ray Equipment Procurement 2026
Frequently Asked Questions (FAQs)
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a dental X-ray unit in 2026? | Dental X-ray systems typically require a stable 110–120V or 220–240V AC power supply, depending on regional standards and equipment class. In 2026, many modern digital units support auto-switching voltage (100–240V) for global compatibility. Always confirm the input voltage, frequency (50/60 Hz), and grounding requirements in the technical specifications. For wall-mounted or panoramic/CBCT units, a dedicated circuit with surge protection is recommended to ensure operational safety and compliance with IEC 60601-1 standards. |
| 2. How can I ensure long-term availability of spare parts for my dental X-ray equipment? | Manufacturers are now required under EU MDR and FDA guidelines to declare spare parts availability for a minimum of 10 years post-discontinuation. When procuring equipment, request a written commitment from the supplier or OEM regarding spare parts support duration. Prioritize brands with local or regional distribution centers, as they typically maintain higher inventory levels of critical components (e.g., X-ray tubes, sensors, collimators). Additionally, verify if firmware updates and software licenses are included in long-term maintenance agreements. |
| 3. What does the installation process involve for a new dental X-ray system, and is professional assistance required? | Installation of dental X-ray units—especially intraoral, panoramic, or CBCT systems—must be performed by certified biomedical technicians or manufacturer-trained engineers. The process includes site evaluation (space, power, shielding), mechanical mounting, electrical connection, radiation safety checks, and calibration. In 2026, most premium systems include remote diagnostic setup and AI-assisted alignment. Ensure your clinic meets structural and radiation shielding regulations (e.g., NCRP Report No. 177) prior to installation. Turnkey solutions from leading vendors often include site preparation guidance and regulatory documentation support. |
| 4. What warranty coverage should I expect when purchasing dental X-ray equipment in 2026? | Standard warranty terms for dental X-ray units in 2026 range from 2 to 5 years, covering parts, labor, and technical support. High-end CBCT and panoramic systems often include a 3-year comprehensive warranty with on-site service response within 48 hours. Verify whether the warranty includes the X-ray tube (a high-cost component), sensor modules, and software updates. Extended warranty plans with predictive maintenance monitoring are increasingly available and recommended for maximizing uptime and ROI. Ensure warranty terms are transferable in case of clinic resale or relocation. |
| 5. Are there any new regulatory or compliance considerations for dental X-ray equipment in 2026? | Yes. As of 2026, all new dental X-ray devices must comply with updated IEC 60601-2-54 (safety for X-ray equipment) and include dose optimization features such as AI-driven exposure control and DICOM calibration. In the U.S., FDA 510(k) clearance remains mandatory, while EU clinics must confirm CE marking under the revised Medical Device Regulation (MDR) 2017/745. Additionally, equipment must support HL7/FHIR integration for EHR compatibility and adhere to local radiation safety protocols (e.g., ALARA principles). Always request compliance documentation and a certificate of conformity at time of purchase. |
Need a Quote for Dental X Ray Equipments?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


