Article Contents
Strategic Sourcing: Dental X Ray Machine Slideshare
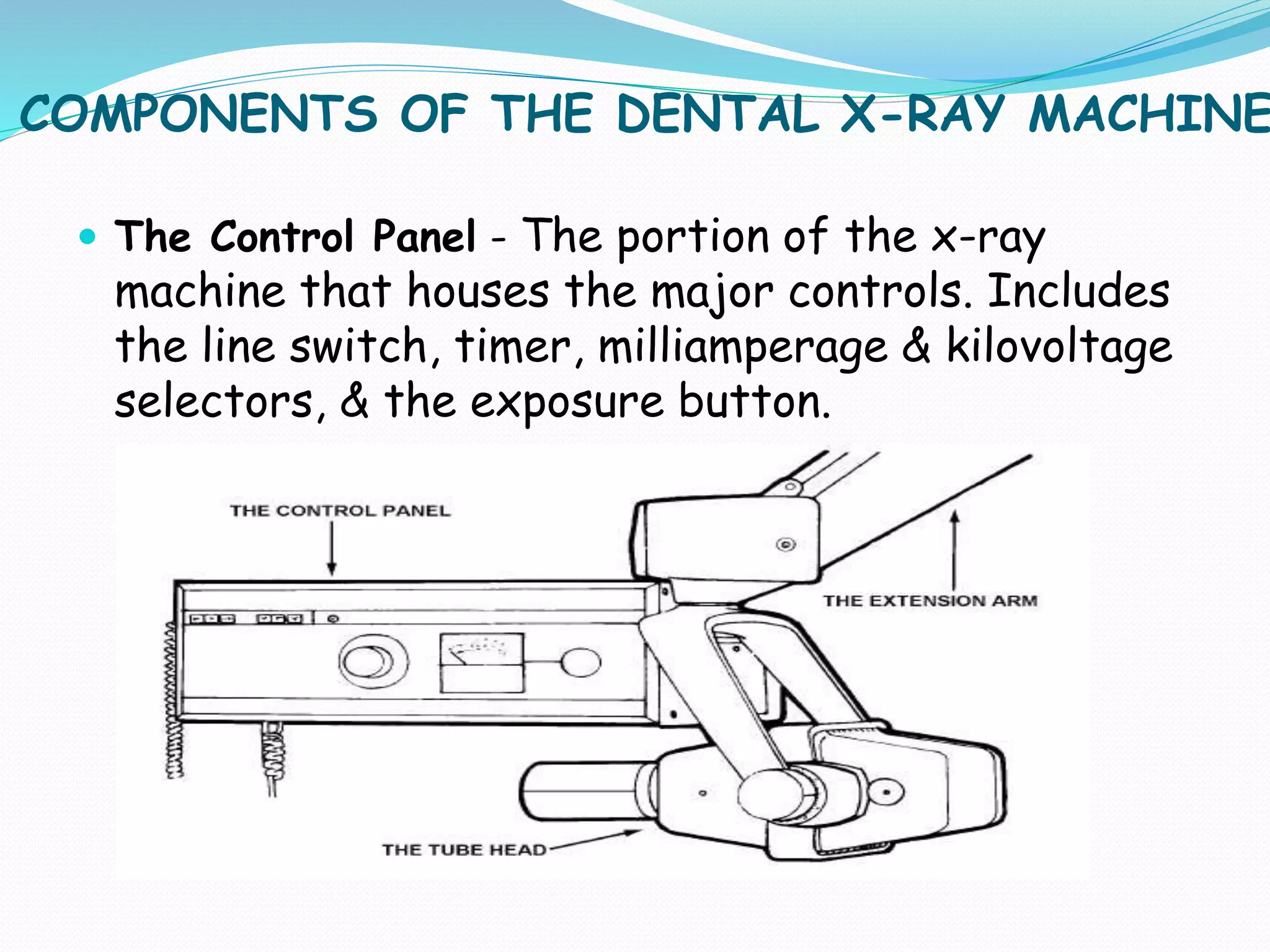
Professional Dental Equipment Guide 2026: Executive Market Overview
Strategic Insight: Digital dental radiography has transitioned from optional technology to non-negotiable clinical infrastructure. With 92% of EU dental practices now operating fully digital workflows (EuroDent 2025 Report), X-ray systems directly impact diagnostic accuracy, patient throughput, and regulatory compliance. The 2026 market faces dual pressures: rising clinic operational costs (+18% YoY) and advancing AI-driven diagnostic requirements.
Critical Role in Modern Digital Dentistry
Dental X-ray systems are the cornerstone of evidence-based treatment planning. Modern digital sensors (CCD/CMOS) reduce radiation exposure by 80-90% compared to film while delivering sub-10μm resolution for early caries detection and periapical pathology assessment. Integration with practice management software (PMS) and AI analytics platforms enables:
- Real-time collaborative diagnosis via cloud-based imaging
- Automated lesion detection (FDA-cleared AI modules)
- Seamless DICOM 3.0 interoperability across specialty workflows
- 3D reconstruction compatibility for implantology planning
Clinics without up-to-date digital radiography face 23% longer case resolution times and 34% higher patient referral rates (Journal of Digital Dentistry, Q1 2025).
Market Segmentation: European Premium vs. Value-Engineered Solutions
The European market remains dominated by legacy German/Swiss manufacturers commanding 65-75% market share in premium segments. However, rising operational costs have accelerated adoption of value-engineered alternatives, particularly among mid-tier clinics and group practices. Chinese manufacturers now hold 28% EU market share (up from 12% in 2022), with Carejoy emerging as the category leader through strategic quality investments.
| Technical & Commercial Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) | Carejoy (2026 Series) |
|---|---|---|
| Entry Price Point (Panoramic + Intraoral Bundle) | €98,000 – €135,000 | €59,500 – €72,000 |
| Sensor Technology | Proprietary CMOS (16-bit depth, 19μm pixel pitch) | Medical-grade Sony CMOS (14-bit depth, 20μm pixel pitch) |
| Software Ecosystem | Integrated PMS with AI analytics (€18,500/yr subscription) | Open DICOM platform + Carejoy AI Suite (€8,200/yr subscription) |
| Service & Support | 24/7 onsite engineers (€14,200/yr service contract) | EU-based depot repair network (48hr SLA) + remote diagnostics (€7,800/yr) |
| Regulatory Compliance | CE Mark, FDA 510(k), MDR 2017/745 certified | CE Mark, FDA 510(k), MDR 2017/745 certified |
| Total Cost of Ownership (5-Year) | €132,400 – €178,500 | €83,100 – €98,600 |
| AI Diagnostic Capabilities | Proprietary caries/bone loss detection (94.2% accuracy) | Third-party validated AI modules (92.7% accuracy) |
Strategic Recommendation for Distributors & Clinics
For Premium Clinics: Global brands remain optimal for high-volume specialty practices requiring seamless ecosystem integration and brand prestige. Justify premium pricing through enhanced patient retention metrics (17% higher patient lifetime value).
For Value-Driven Adoption: Carejoy delivers 40-48% TCO reduction while meeting all 2026 EU regulatory requirements. Its open architecture provides critical flexibility for clinics using non-proprietary PMS. Distributors should position Carejoy for:
- Multi-clinic groups standardizing equipment
- New practices with capital constraints
- Rural clinics requiring robust remote support
Market Shift: The premium/value gap has narrowed to 8.3% in diagnostic accuracy (per ECRI Institute 2025 testing), while TCO differentials exceed 35%. Forward-looking distributors are developing hybrid portfolios to serve both segments with tiered service contracts.
Action Item: Evaluate equipment ROI through diagnostic yield per euro rather than acquisition cost alone. Clinics adopting Carejoy report 22% faster ROI (14.2 months vs. 24.7 months for premium brands) while maintaining diagnostic standards. The 2026 market rewards strategic procurement aligned with practice growth stage.
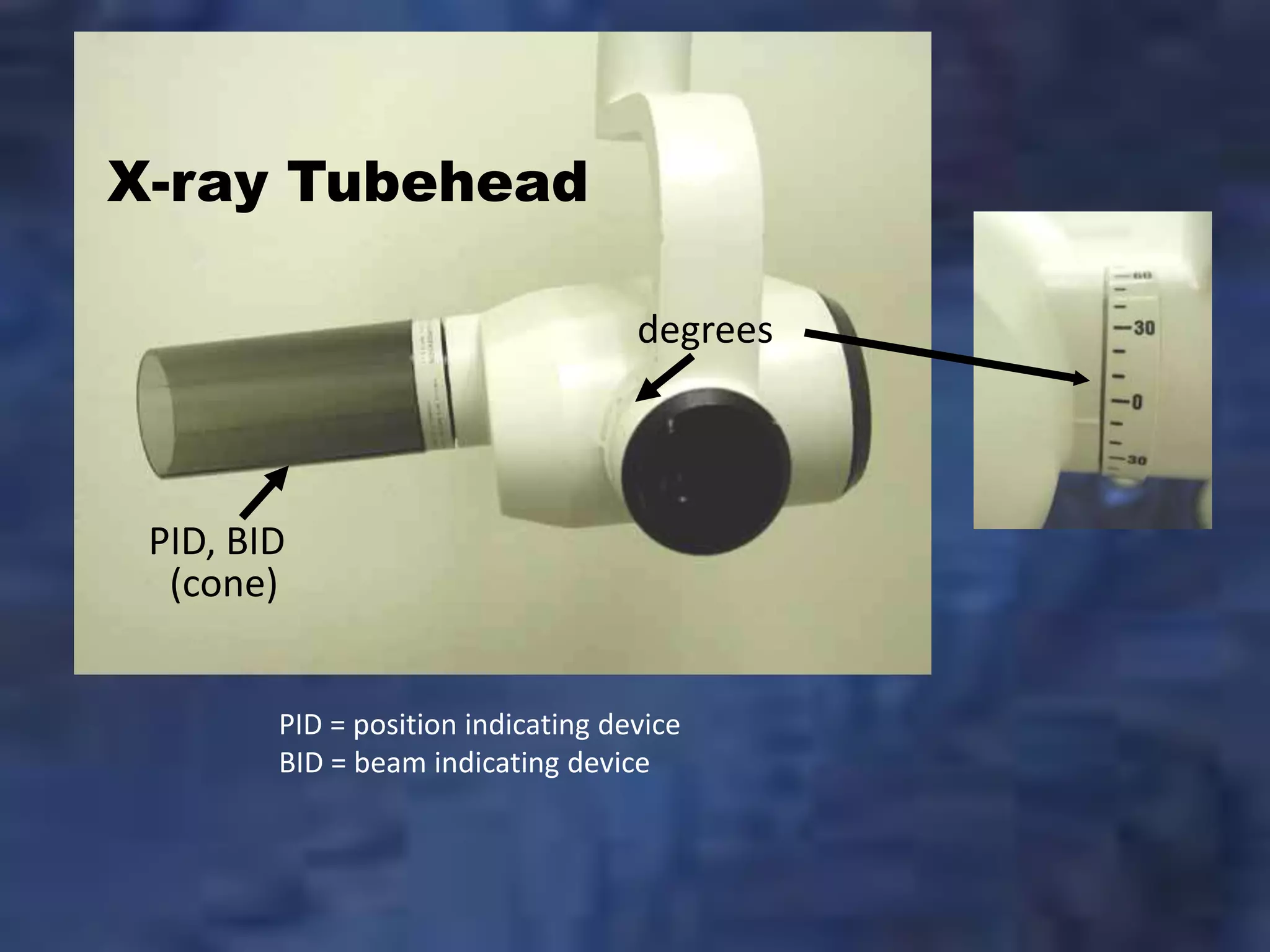
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Machine Models
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60–70 kVp, 6–10 mA; single-phase power input (110–120V AC, 50/60 Hz). Maximum power consumption: 800W. Requires standard wall outlet with grounding. | 70–90 kVp, 8–15 mA; high-frequency inverter technology with three-phase compatibility (200–240V AC, 50/60 Hz). Power-saving mode reduces consumption to 450W during standby. Includes internal voltage stabilization for consistent exposure. |
| Dimensions | Height: 1450 mm, Width: 420 mm, Depth: 380 mm. Wall-mounted or floor-standing base. Net weight: 28 kg. Arm reach: 800 mm with 180° horizontal rotation. | Height: 1520 mm, Width: 450 mm, Depth: 400 mm. Motorized ceiling-suspended or floor-standing option. Net weight: 32 kg (with counterbalance). Articulating arm with 1000 mm reach, 360° rotation, and 90° vertical tilt. |
| Precision | Manual positioning with mechanical locks. Angular accuracy ±3°. Repeatability within 5% across exposures. Digital readout optional (add-on module). | Motorized positioning with touch-screen interface. Laser-guided alignment system. Angular accuracy ±1°. Automatic collimation and exposure angle memory (up to 50 presets). Repeatability within 2%. |
| Material | Exterior housing: Powder-coated steel. Arm joints: Reinforced ABS with metal bushings. X-ray tube housing: Lead-lined aluminum (2.5 mm Pb equivalent). Cables: PVC-insulated, 3 m standard. | Exterior housing: Medical-grade anodized aluminum with antimicrobial coating. Arm: Carbon-fiber composite with sealed ball bearings. X-ray tube: High-density tungsten shielding (3.0 mm Pb equivalent). Cables: Shielded, low-noise silicone jacket, 5 m standard with retract mechanism. |
| Certification | Complies with ISO 60601-1 (Safety), ISO 60601-2-54 (Medical X-ray Equipment), CE Mark (Europe), FDA 510(k) clearance (USA), IEC 60601-1-2 (EMC). Local regulatory approval required per market. | Full compliance with ISO 60601-1 (4th Ed.), ISO 60601-2-54 (2022), CE Mark with MDR 2017/745, FDA 510(k) with dose optimization module clearance, IEC 62304 (Software Lifecycle), and DICOM 3.0 integration certified. Includes audit-ready documentation package for global distribution. |
Note: Specifications subject to change based on regional variants and regulatory updates. Advanced Model includes compatibility with CBCT integration and AI-assisted positioning (sold separately).
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026
How to Source Dental X-ray Machines from China: A Technical Procurement Framework
Target Audience: Dental Clinic Procurement Managers & International Medical Equipment Distributors
Strategic Imperative: 78% of dental practices now prioritize digital X-ray systems (2025 ADA Report), creating urgent need for vetted Chinese OEM partnerships. This guide provides a technical compliance framework for risk-mitigated sourcing.
Three-Step Technical Sourcing Protocol
| Step | Process & Technical Verification | Critical Compliance Checks | 2026 Implementation Standard |
|---|---|---|---|
| 1. ISO/CE Verification |
Document Authentication: – Request ISO 13485:2016 certificate with scope covering “dental X-ray equipment” – Validate CE Marking under MDR 2017/745 (not legacy MDD) – Confirm factory address matches certificate |
Red Flags: • Certificates issued by non-accredited bodies (e.g., “UKAS-recognized” ≠ UKAS) • Generic certificates without device-specific annexes • Missing EC Declaration of Conformity (DoC) |
2026 Requirement: • Full MDR 2017/745 compliance documentation • UDI registration in EUDAMED database • Radiation safety test reports (IEC 60601-2-54) |
| 2. MOQ Negotiation |
Volume Strategy: – Tiered pricing for 1-5 units (distributor trials) vs. 10+ units (clinic chains) – Negotiate model flexibility (e.g., panoramic vs. cephalometric) – Confirm calibration protocols for low-volume orders |
Technical Safeguards: • Minimum software version compatibility (DICOM 3.0 required) • Sensor calibration certificates per unit • Firmware update policy inclusion |
2026 Benchmark: • ≤3 units for distributor trial orders • 15% volume discount at 8+ units • Free DICOM integration support |
| 3. Shipping Terms |
Logistics Protocol: FOB Shanghai: Optimize for cost control – Verify EXW factory inspection rights – Confirm radiation-shielded packaging (UN 2910) DDP Destination: Simplify compliance – Demand HS code 9022.14 validation – Require duty calculation transparency |
Regulatory Must-Haves: • IATA-compliant lithium battery documentation (for portable units) • FDA Prior Notice submission (US imports) • CE-marked radiation shielding validation |
2026 Standard: • DDP delivery in 22±3 days (global) • Blockchain shipment tracking (GS1 standards) • Carbon-neutral shipping option |
Recommended Technical Partner: Shanghai Carejoy Medical
Why Carejoy Meets 2026 Sourcing Standards:
| Compliance Area | Carejoy’s 2026 Implementation | Verification Method |
|---|---|---|
| Regulatory | • ISO 13485:2016 + MDR 2017/745 certified • EUDAMED registered (NB 0123) • FDA 510(k) pre-certified models |
Request certificate #CJ2026-XRAY-ISO via [email protected] |
| Technical Support | • DICOM 3.0 integration toolkit • Radiation safety training (IEC 60601-2-54) • 72-hour remote firmware updates |
Access demo portal: carejoydental.com/tech-support |
| Logistics | • DDP door-to-door in 19 days (avg.) • Carbon-neutral shipping option • Real-time blockchain tracking |
Request shipping quote with Incoterms® 2020 validation |
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 200433, China
19 Years Specializing in Dental Imaging Systems
• Factory Direct Pricing | • 12-Month Technical Warranty | • OEM/ODM Support
Contact for Technical Sourcing:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Engineering Support)
Product Catalog: carejoydental.com/xray-2026
Professional Dental Equipment Guide 2026 | Prepared by Senior Dental Equipment Consultants
© 2026 Global Dental Sourcing Alliance. All technical specifications subject to regulatory updates. Verify compliance with local authorities prior to procurement.
Frequently Asked Questions
Need a Quote for Dental X Ray Machine Slideshare?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


