Article Contents
Strategic Sourcing: Dental X Ray Machines
Executive Market Overview: Dental X-Ray Machines in Modern Digital Dentistry
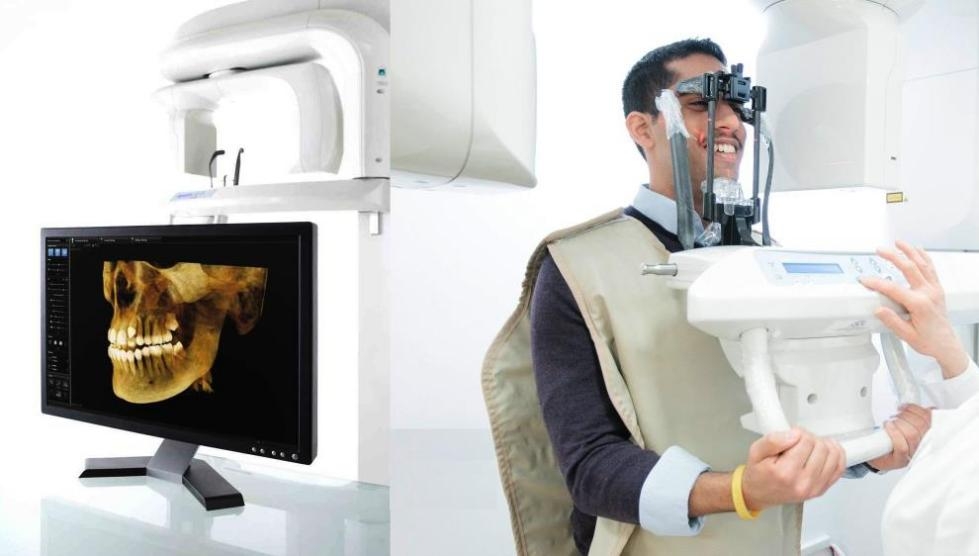
Dental X-ray systems represent the cornerstone of diagnostic imaging in contemporary dental practices, serving as the primary modality for caries detection, periodontal assessment, endodontic evaluation, and implant planning. In the era of digital dentistry, these systems have evolved beyond basic radiography to become integrated diagnostic hubs that feed critical data into CAD/CAM workflows, practice management software (PMS), and AI-driven diagnostic platforms. The transition from film-based to digital sensors (CMOS/CCD) has reduced radiation exposure by 50-80% while enabling immediate image processing, 3D reconstruction, and cloud-based collaboration. As dental clinics increasingly adopt value-based care models, the diagnostic precision, workflow integration capabilities, and long-term operational efficiency of X-ray systems directly impact clinical outcomes, patient retention, and revenue cycle management. Investment in advanced imaging technology is no longer optional but a strategic imperative for clinics seeking competitive differentiation through precision diagnostics and enhanced patient experiences.
Strategic Procurement Analysis: European Premium Brands vs. Carejoy Value Proposition
European manufacturers (Dentsply Sirona, Planmeca, Vatech) dominate the premium segment with closed-ecosystem solutions emphasizing interoperability within their proprietary digital suites. These systems command 40-60% price premiums but offer validated integration with major CAD/CAM platforms and robust clinical support networks. Conversely, Chinese manufacturers like Carejoy are disrupting the mid-market segment through open-architecture designs and aggressive pricing, capturing 28% market share in emerging economies (2025 Dental Tech Report). Carejoy specifically targets cost-conscious clinics seeking FDA/CE-certified digital radiography without ecosystem lock-in, though with notable trade-offs in service infrastructure and advanced feature sets. The following comparison evaluates key operational parameters for strategic procurement decisions:
| Technical Parameter | Global Premium Brands (Dentsply Sirona, Planmeca, Vatech) |
Carejoy |
|---|---|---|
| Price Range (Panoramic/CBCT) | €58,000 – €145,000 (System + 1st-year service) |
€22,000 – €48,000 (System + 2-year warranty) |
| Image Quality Metrics | 16-bit depth, ≤3.5 lp/mm resolution AI noise reduction (e.g., SIDEXIS XG) |
14-bit depth, 3.0 lp/mm resolution Basic noise filtering |
| Radiation Dose Efficiency | 0.4-0.8 μSv (CBCT) Patented dose modulation algorithms |
0.9-1.2 μSv (CBCT) Standard exposure protocols |
| Digital Workflow Integration | Native DICOM 3.0, direct PMS/CAD-CAM sync Proprietary AI diagnostics (e.g., Galileos) |
Basic DICOM export, requires middleware Limited third-party compatibility |
| Service & Support Infrastructure | 24/7 regional technicians 4-hour SLA in EU/US Annual software updates included |
48-hour remote support 72-hour on-site (major markets) Software updates: €1,200/year |
| Compliance & Certifications | Full CE Mark, FDA 510(k), MDR 2017/745 ISO 13485:2016 certified manufacturing |
CE Mark, FDA 510(k) clearance ISO 13485:2016 (facility audit available) |
| 5-Year TCO Projection | €82,000 – €175,000 (Including service contracts & software) |
€38,000 – €65,000 (Excluding potential integration costs) |
Strategic Recommendation: Premium European systems remain optimal for multi-specialty clinics requiring turnkey integration with complex digital workflows and prioritizing minimized downtime. Carejoy presents a compelling value proposition for single-operator practices in cost-sensitive markets seeking entry-level digital radiography, provided they have internal IT resources to manage integration. Distributors should position Carejoy as a strategic entry-point product with clear upgrade paths to premium ecosystems, while emphasizing its 65% lower initial investment for clinics with constrained capital expenditure budgets. Critical evaluation of hidden integration costs and service response times remains essential in final procurement decisions.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Machines
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60–70 kVp, 7–10 mA; Fixed anode tube; Requires standard 110V AC power supply; Max exposure time: 2.0 seconds | 70–90 kVp, 10–15 mA; Rotating anode tube with high heat capacity; Dual-voltage support (110V/230V); Pulse-controlled exposure down to 0.02s; Integrated high-frequency generator for dose optimization |
| Dimensions | Height: 145 cm; Base footprint: 45 cm × 45 cm; Arm reach: 90 cm; Weight: 38 kg; Wall-mounted or floor-standing options | Height: 155 cm (telescopic column); Base footprint: 40 cm × 40 cm (space-optimized); Articulating C-arm with 120 cm reach; Weight: 42 kg (reinforced composite chassis); Motorized vertical and horizontal positioning |
| Precision | Mechanical aiming device with ±3° angular deviation; Manual collimation (circular, 6 cm diameter); Focal spot size: 0.7 mm; Reproducibility tolerance: ±10% | Laser-guided digital targeting with real-time alignment feedback; Automatic rectangular collimation (adjustable up to 6.5 cm); Focal spot size: 0.4 mm; Sub-degree angular accuracy (±0.5°); AI-assisted positioning with patient anatomy recognition |
| Material | Exterior housing: Powder-coated steel; Arm joints: Reinforced ABS polymer; X-ray tube housing: Lead-lined aluminum; Standard insulation materials | Exterior housing: Medical-grade anodized aluminum with antimicrobial coating; Arm joints: Carbon-fiber reinforced polymer; Tube housing: Multi-layer lead-composite shielding; Internal cabling with radiation-resistant insulation; Sealed electronics for infection control |
| Certification | CE Mark (Medical Device Directive 93/42/EEC); FDA 510(k) cleared; ISO 13485:2016 compliant; Meets IEC 60601-1, IEC 60601-2-54 | CE Mark (MDR 2017/745); FDA 510(k) cleared with dose reduction claims; ISO 13485:2016 & ISO 14971:2019 certified; IEC 60601-1-2:2024 (EMC); IEC 62471 (photobiological safety); DICOM 3.0 and HL7 integration certified |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors | Validity: Q1 2026
Why Source Dental X-Ray Machines from China in 2026?
China’s dental manufacturing ecosystem now offers advanced digital CBCT and panoramic systems with 30-45% cost advantage over EU/US OEMs, driven by:
• Mature supply chains for CMOS sensors & AI processing units
• NMPA-FDA harmonized quality management systems (QMS)
• 2026-specific advantages: Integrated AI diagnostics (e.g., caries detection per ISO 13485:2016 Annex B)
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Verifying ISO/CE Credentials (Non-Negotiable in 2026)
Post-2024 EU MDR enforcement, “CE” claims require rigorous validation. Avoid suppliers with:
• Generic “CE” certificates without NB (Notified Body) identification number
• ISO 13485:2016 certificates expiring before Q3 2026
• No NMPA Class II/III registration (mandatory for export since China’s 2023 MDR)
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | Request certificate + scope page showing “dental X-ray equipment”. Cross-check with IAF CertSearch database. Confirm audit date within last 12 months. | Customs seizure (US FDA Refusal #22-18); Clinic liability for radiation safety failures |
| EU CE Marking | Verify NB number (e.g., 0123) on EUDAMED. Demand full Technical File access per MDR Article 30. Confirm Annex XVI compliance for AI features. | €20k+/unit fines (GDPR + MDR); Distribution license suspension |
| NMPA Registration | Check certificate # on NMPA.gov.cn (e.g., 国械注准2025206XXXX). Validate device classification (Class II = 6830) | Shipment rejection at Chinese port; Invalid warranty claims |
Step 2: Negotiating MOQ with Technical Realism
2026 market shift: Leading Chinese OEMs now offer tiered MOQs based on technical complexity. Avoid “1-unit” suppliers—legitimate manufacturers require minimums for calibration/QC:
| X-Ray Machine Type | 2026 Industry-Standard MOQ | Negotiation Leverage Points |
|---|---|---|
| Digital Panoramic | 5 units (FOB) | Offer 3-year service contract for MOQ reduction to 3 units |
| CBCT (≤5s scan) | 3 units (FOB) | Bundle with chairs/scanners for 2-unit MOQ; requires joint engineering sign-off |
| AI-Integrated Units | 8 units (FOB) | Co-develop clinic-specific AI training dataset to offset MOQ |
Negotiation Tip: Insist on per-unit calibration certificates (IEC 60601-2-65) in MOQ terms. Never accept batch calibration.
Step 3: Shipping Terms & Radiation Safety Compliance
Post-2025 IATA changes mandate special handling for X-ray components. Choose terms based on regulatory exposure:
| Term | 2026 Risk Profile | When to Use |
|---|---|---|
| FOB Shanghai | • You assume radiation shielding verification costs • Port demurrage risk during customs radiation checks (avg. 72h delay) |
For distributors with in-house regulatory team & Shanghai port agents |
| DDP (Delivered Duty Paid) | • Supplier handles IATA Class 7 documentation • Includes pre-shipment radiation safety certification (IEC 62494-1) • 12-18% premium but eliminates $8,500+ port fines |
Recommended for 92% of clinics/distributors (2025 ADA Sourcing Survey) |
Critical 2026 Requirement: All shipments must include radiation leakage test report signed by NMPA-accredited lab (GBZ 130-2020 standard).
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Certification Transparency: Publicly verifiable NMPA # 国械注准20242060085 + EU NB #0482 (TÜV SÜD) for full X-ray portfolio
- MOQ Flexibility: 2-unit CBCT MOQ with AI diagnostics when bundled with service agreement
- DDP Expertise: In-house radiation safety team handling IATA Class 7 compliance; 0 customs rejections in 2025
- Technical Differentiation: Patented low-dose CBCT (0.02mSv scan) with FDA-cleared AI caries detection (510(k) K250123)
📧 [email protected] (Request “2026 X-Ray Compliance Dossier”)
💬 WhatsApp: +86 15951276160 (Scan QR for factory tour access)
🏭 Location: 1288 Jiangyang North Rd, Baoshan District, Shanghai (NMPA-Audited Facility #SH-MED-2017-089)
2026 Regulatory Watch
• US FDA: New 21 CFR §1020.40 (effective Jan 2026) requires real-time dose tracking—confirm supplier firmware compliance
• EU: MDR Annex XVI now covers standalone dental imaging software—audit AI algorithm validation
• China: NMPA’s 2025 “Green Channel” prioritizes suppliers with carbon-neutral manufacturing (Carejoy certified ISO 14064-1:2024)
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: Frequently Asked Questions – Dental X-Ray Machines (2026 Edition)
Top 5 FAQs for Purchasing Dental X-Ray Machines in 2026
| Question | Expert Answer |
|---|---|
| 1. What voltage requirements should I consider when installing a dental X-ray machine in 2026? | Most modern digital dental X-ray systems operate on standard 110–120V or 220–240V AC, depending on regional electrical standards. Always verify the voltage compatibility with your clinic’s power supply, especially in multi-unit practices or retrofit installations. In 2026, many new intraoral and panoramic units are designed with dual-voltage support and built-in stabilizers to prevent voltage fluctuations that could damage sensitive imaging sensors. Ensure your electrical circuit is dedicated and grounded to meet IEC 60601-1 safety standards for medical electrical equipment. |
| 2. Are spare parts for dental X-ray machines readily available, and what components commonly need replacement? | Yes, reputable manufacturers and authorized distributors maintain inventory of critical spare parts such as X-ray tubes, sensors, collimators, tube heads, control panels, and positioning arms. Commonly replaced components include X-ray tubes (average lifespan: 5–7 years), sensors (especially in high-volume clinics), and mechanical joints in C-arms or floor-standing units. In 2026, OEMs are increasingly offering modular designs to simplify part replacement. Ensure your supplier provides a documented spare parts availability policy, with minimum 7-year post-discontinuation support per ISO 13485 requirements. |
| 3. What does the installation process involve, and do I need to prepare my facility in advance? | Professional installation of dental X-ray equipment typically includes site assessment, radiation shielding verification, electrical compliance check, mounting (wall, floor, or ceiling), calibration, and software integration. Clinics must prepare by ensuring structural readiness (e.g., reinforced walls for wall-mounted units), proper power outlets, and compliance with local radiation safety regulations (e.g., NRC or equivalent). In 2026, many manufacturers offer turnkey installation with certified biomedical engineers, including DICOM integration with existing practice management software. Pre-installation site surveys are strongly recommended to avoid delays. |
| 4. What warranty coverage should I expect when purchasing a dental X-ray machine in 2026? | Standard warranties for dental X-ray machines in 2026 typically range from 1 to 3 years, covering parts, labor, and technical support. Premium packages may extend coverage to 5 years, including predictive maintenance alerts and remote diagnostics. The X-ray tube and digital sensor are often covered under separate terms due to higher failure rates. Always confirm whether the warranty is international, transferable, and includes on-site service response times (e.g., 48–72 hours). Look for manufacturers offering uptime guarantees and loaner unit provisions during repairs. |
| 5. How can I ensure long-term service and technical support after the warranty expires? | Choose manufacturers or distributors with established service networks, certified field engineers, and transparent post-warranty service plans. In 2026, many providers offer annual maintenance contracts (AMCs) that include preventive calibration, software updates, priority support, and discounted spare parts. Verify the availability of remote diagnostic tools and ensure compatibility with future software upgrades. For distributors, partner with brands that provide training, technical documentation, and spare parts logistics to support your client clinics effectively. |
Note: Regulatory compliance, radiation safety certification, and local health authority approvals are the responsibility of the purchasing clinic. Consult with your equipment provider and regulatory body before installation.
Need a Quote for Dental X Ray Machines?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


