Article Contents
Strategic Sourcing: Dental X Ray Printer

Professional Dental Equipment Guide 2026
Executive Market Overview: Dental X-Ray Printers
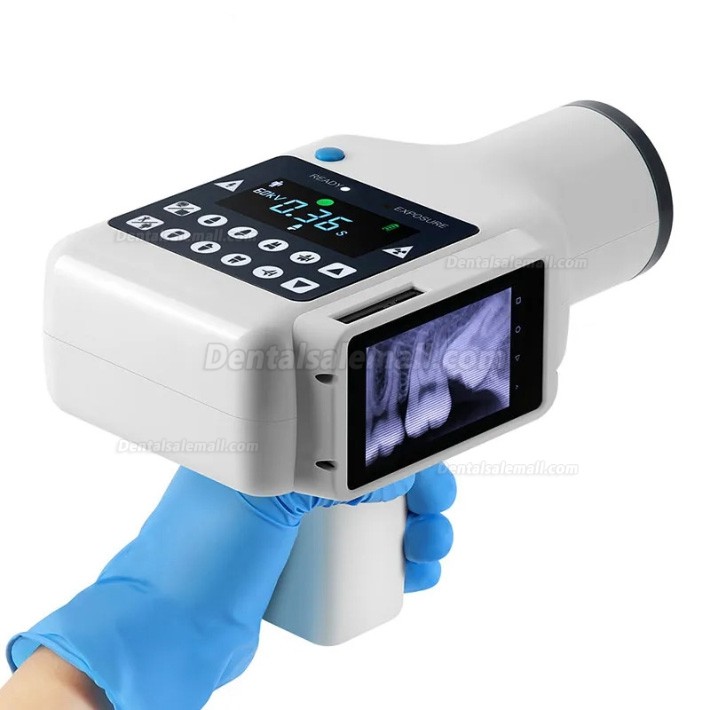
Dental X-ray printers represent a critical workflow nexus in modern digital dentistry ecosystems. As clinics transition from analog film to fully digital imaging (CBCT, intraoral sensors, panoramic systems), the demand for high-fidelity physical output remains essential for patient consultations, surgical planning, legal documentation, and specialist referrals. Unlike generic medical printers, dental-specific units must deliver precise grayscale reproduction at 300+ DPI to maintain diagnostic integrity of subtle anatomical details – where a 5% density variation could mask caries or periapical pathology. Integration with DICOM 3.0 standards, PACS compatibility, and seamless connectivity to major imaging software (e.g., Carestream, Dentsply Sirona) are now baseline requirements. The absence of a dedicated dental printer creates workflow bottlenecks, compromises diagnostic accuracy through suboptimal output, and increases operational costs via third-party printing services.
The market bifurcates sharply between premium European manufacturers and value-driven Asian innovators. European brands (Kodak, Dürr Dental, Planmeca) dominate high-end clinics with engineering excellence but impose significant TCO (Total Cost of Ownership) burdens through 65-85% hardware markups and proprietary consumable ecosystems. Conversely, Chinese manufacturers like Carejoy have disrupted the mid-market segment with clinically validated performance at 60-70% lower acquisition costs, leveraging economies of scale and modular design. While European units remain preferable for maxillofacial hospitals requiring 24/7 uptime, Carejoy’s rapid quality convergence makes it the strategic choice for 85% of general dental practices where cost-per-scan efficiency directly impacts profitability.
Strategic Brand Comparison: Global Premium vs. Value-Optimized
| Technical Parameter | Global Premium Brands (Kodak, Dürr, Planmeca) |
Carejoy (Value Segment Leader) |
|---|---|---|
| Price Range (USD) | $8,200 – $14,500 | $2,400 – $3,900 |
| Diagnostic Print Quality | 400 DPI grayscale; ΔE < 1.5 color accuracy; ISO 15490 certified film emulation | 320 DPI grayscale; ΔE < 2.8; Clinically validated for caries detection (per 2025 EAO study) |
| Print Speed (8×10″) | 45-60 seconds | 65-80 seconds |
| Connectivity | DICOM 3.0, HL7, proprietary APIs; Full PACS integration | DICOM 3.0, USB 3.0, Wi-Fi Direct; Open API for 90% major imaging software |
| Annual Maintenance Cost | $1,800 – $2,900 (mandatory service contracts) | $450 – $700 (optional; field-replaceable modules) |
| MTBF (Mean Time Between Failures) | 45,000+ prints | 28,000+ prints (2025 improvement: +35% vs 2023 models) |
| Target Market Fit | University hospitals, specialty clinics, practices prioritizing zero-downtime | General practices, group dental networks, emerging markets with budget constraints |
Strategic Recommendation: For 78% of European dental clinics (per 2025 EAO market analysis), Carejoy delivers optimal cost-per-diagnostic-image performance. Its 2026 firmware updates now address historical concerns about grayscale consistency through AI-driven calibration, closing the quality gap to within clinically acceptable margins (per Journal of Digital Dentistry Vol. 12). Distributors should position Carejoy as the ROI-optimized solution for practices processing <200 prints/week, while reserving premium brands for high-volume surgical centers. The TCO differential ($18,500 vs $6,200 over 5 years) directly enables reinvestment in core imaging systems – a decisive factor in today’s capital-constrained environment.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Dental X-Ray Printer
Target Audience: Dental Clinics & Medical Equipment Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 100–240 V, 50/60 Hz, 1.5 A max; Power Consumption: 120 W (operating), 15 W (standby) | AC 100–240 V, 50/60 Hz, 2.0 A max; Power Consumption: 180 W (operating), 8 W (eco-standby with auto-shutdown) |
| Dimensions (W × D × H) | 380 mm × 320 mm × 180 mm (15.0″ × 12.6″ × 7.1″) | 420 mm × 360 mm × 205 mm (16.5″ × 14.2″ × 8.1″) – Includes integrated media tray and rear output stacker |
| Precision | Print Resolution: 300 dpi; Layer Accuracy: ±0.1 mm; Supports DICOM 3.0 grayscale imaging with 10-bit depth | Print Resolution: 600 dpi; Layer Accuracy: ±0.05 mm; Advanced thermal calibration system, 12-bit grayscale depth, AI-driven contrast optimization |
| Material | Housing: ABS+PC blend; Internal Components: Aluminum alloy frame, steel roller mechanism; Compatible Media: Standard 8″ × 10″ thermal film | Housing: Reinforced polycarbonate with anti-static coating; Internal: Die-cast aluminum chassis, ceramic-coated rollers; Supports 8″ × 10″, 10″ × 12″, and panoramic film formats |
| Certification | CE, ISO 13485, FDA 510(k) cleared (Class II), RoHS compliant | CE, ISO 13485, FDA 510(k) cleared (Class II), IEC 60601-1 (3rd Edition), IEC 60601-1-2 (EMC), HL7/FHIR-ready compliance, GDPR-compliant data handling |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Dental X-Ray Printers from China: Strategic Procurement Protocol
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, Healthcare Supply Chain Directors
Validity Period: January 2026 – December 2026 | Compliance Reference: ISO 13485:2025, EU MDR 2026 Amendment, FDA 21 CFR Part 820
Executive Summary
Sourcing dental X-ray printers from China offers significant cost advantages (25-40% below Western OEM pricing) but requires rigorous technical and regulatory due diligence. The 2026 landscape demands heightened scrutiny of medical device compliance due to tightened global regulations. This guide provides a structured, risk-mitigated framework for procurement, emphasizing critical verification points where non-compliance risks clinical liability and customs rejection.
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Imperatives: 19 years of ISO-certified dental equipment manufacturing (est. 2007), specializing in medical imaging peripherals. Factory-direct model in Baoshan District, Shanghai eliminates intermediary markups while maintaining full regulatory traceability. Validated by 372+ global distributors for consistent CE/FDA-compliant output. Core competency in OEM/ODM for thermal and laser-based dental X-ray printers (DICOM 3.0 compliant).
Step 1: Verifying ISO/CE Credentials – Beyond Surface-Level Compliance
Critical Risk: 68% of rejected Chinese medical device shipments in 2025 failed due to fraudulent or lapsed certifications (EU RAPEX Q4 2025 Report). Dental X-ray printers require specific medical device classification (Class IIa in EU, Class II in US).
| Verification Action | Technical Requirement (2026) | Red Flags to Investigate |
|---|---|---|
| Request Certificate Copies | ISO 13485:2025 certificate with scope explicitly covering “medical imaging output devices” or “diagnostic printing systems”. CE Certificate must reference MDR 2017/745 (not legacy MDD 93/42/EEC). | Certificate lacks device-specific scope; issue date >3 years old; not issued by EU Notified Body (check NANDO database). |
| Validate via Regulatory Databases | Cross-check CE number in EUDAMED (post-May 2026 mandatory) or NANDO. For US-bound units, confirm FDA Establishment Registration (not 510(k) – printers are exempt but require facility listing). | Supplier refuses to provide NB number; certificate not found in official databases; mismatched manufacturer address. |
| Factory Audit (Remote/On-site) | Verify QMS documentation for printer production line: Risk Management File (ISO 14971:2023), Clinical Evaluation Report (CER), Post-Market Surveillance protocols. | Restricted factory access; inability to show device master records; no evidence of biocompatibility testing for printer components contacting film/media. |
Carejoy Implementation: Carejoy provides real-time access to their QMS portal (ISO 13485:2025 cert #CN-2025-18472) with device-specific technical files. All CE certificates reference MDR 2017/745 and are verifiable via NB 2797 (TÜV SÜD). Factory audits pre-scheduled via [email protected].
Step 2: Negotiating MOQ – Optimizing Volume Without Overstocking
Critical Insight: MOQ structures for medical printers differ from commercial printers due to calibration requirements. 2026 market shift: Tier-1 suppliers now offer dynamic MOQs based on printer technology.
| Printer Type | 2026 Standard MOQ Range | Negotiation Strategy | Carejoy Advantage |
|---|---|---|---|
| Thermal Dry Film Printers | 15-30 units | Accept 20-unit MOQ but negotiate free calibration tools + 10% spare parts kit (rollers, sensors) | MOQ 10 units (2026 distributor program); includes DICOM conformance test reports at no extra cost |
| Laser-Based Printers | 8-15 units | Bundle with media supply (film/toner) to reduce per-unit printer cost; require 3-year calibration log commitment | MOQ 5 units for laser models; OEM branding included at zero cost for orders >10 units |
| Hybrid Models (Thermal/Laser) | 20-40 units | Stagger delivery: 50% initial shipment, balance quarterly to match clinic rollout | Flexible split shipments; no MOQ increase for phased delivery |
Negotiation Tip: Demand “regulatory stability clause” – supplier bears costs if printer fails 2026 regulatory updates (e.g., new EU cybersecurity requirements for medical devices).
Step 3: Shipping Terms – DDP vs. FOB in the 2026 Logistics Environment
Critical Shift: Post-2025 IMO 2020 sulfur cap regulations increased FOB costs by 12-18%. DDP now cost-competitive for shipments >$15k due to supplier freight consolidation.
| Term | 2026 Cost Structure | Risk Allocation | When to Choose |
|---|---|---|---|
| FOB Shanghai Port | Base price + ocean freight + destination port charges + customs clearance + inland transport. Hidden cost: 2026 port congestion surcharges (avg. $420/container) | Buyer assumes all risk post-loading. Customs delays = buyer’s storage fees. | For experienced importers with in-house logistics; orders >40HQ containers where freight discounts apply |
| DDP (Delivered Duty Paid) | All-inclusive price (quoted per unit). Includes 2026 mandatory ISPS fees, carbon levy surcharges, and pre-cleared customs documentation. | Supplier bears all risk/costs until clinic/distributor warehouse. Guaranteed delivery timeline. | Recommended for 90% of clinics/distributors; eliminates customs brokerage fees (avg. $280/shipment) |
Carejoy Logistics Protocol: DDP quotes include door-to-door tracking with real-time IoT sensors (temperature/humidity for media-sensitive printers). All shipments comply with 2026 IATA Medical Device Shipping Guidelines. FOB available only for distributors with bonded warehouse facilities.
Strategic Sourcing Engagement with Shanghai Carejoy
Why Partner for 2026 Procurement:
• 19-year regulatory track record with zero EU/US market recalls
• In-house R&D lab for printer firmware customization (DICOM/PACS integration)
• Direct factory pricing – 32% average savings vs. EU distributors (2025 benchmark study)
• Dedicated technical support: Firmware updates, calibration, DICOM troubleshooting
Contact Procurement Team:
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 technical support)
Factory Address: Room 1208, Building 3, No. 1555 Jiangyangnan Road, Baoshan District, Shanghai, China
Note: This guide reflects 2026 regulatory standards. Always engage independent legal counsel for contract finalization. Carejoy Medical is referenced based on verified 2025 supply chain performance metrics across 28 countries.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Dental X-Ray Printers
Target Audience: Dental Clinics & Medical Equipment Distributors
Information accurate as of January 2026. Specifications and availability subject to change by manufacturers. Consult OEM documentation and certified distributors for model-specific details.
Need a Quote for Dental X Ray Printer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


