Article Contents
Strategic Sourcing: Formlabs Form 3B 3D Printer

Professional Dental Equipment Guide 2026: Executive Market Overview
Formlabs Form 3B+ 3D Printer – Strategic Imperative for Digital Dentistry
Market Context: The European dental 3D printing market is projected to reach €1.8B by 2026 (CAGR 14.2%), driven by the irreversible shift toward digital workflows. Intraoral scanning adoption now exceeds 78% among EU clinics, creating urgent demand for reliable, clinic-integrated additive manufacturing. The Formlabs Form 3B+ has emerged as the de facto standard for mid-to-high volume digital dental production, representing 32% of professional dental printer installations in Western Europe.
Criticality in Modern Workflows: The Form 3B+ is not merely a production tool but a clinical throughput accelerator. Its Low Force Stereolithography (LFS) technology delivers the sub-25μm accuracy required for critical applications: surgical guides (ISO 13485 certified), crown & bridge frameworks, and precision denture bases. Integration with major CAD platforms (exocad, 3Shape) via open APIs enables seamless DICOM-to-print workflows, reducing lab dependency by 65% and enabling same-day restorations. Crucially, its validated biocompatible material ecosystem (e.g., Dental SG, Denture Base) meets EU MDR 2017/745 requirements – a non-negotiable for clinical deployment.
Strategic Procurement Landscape: Clinics face a bifurcated market: Premium European/US brands (Formlabs, EnvisionTEC) command €12,000-€22,000 with proven clinical validation, versus cost-optimized Chinese alternatives (Carejoy, Phrozen) at €4,500-€7,500. While price pressure intensifies, the Total Cost of Ownership (TCO) analysis reveals critical differentiators: failure rates in dental-specific applications exceed 22% for unvalidated Chinese units versus <3% for Form 3B+, directly impacting chairtime utilization and remakes. Distributors must counsel clients on clinical risk mitigation versus initial capex savings.
Global Dental 3D Printer Comparison: Premium vs. Value Segment (2026)
| Parameter | Global Brands (Formlabs/EnvisionTEC) | Carejoy (Representative Value Segment) | Clinical Impact Assessment |
|---|---|---|---|
| Base Price (EUR) | €14,500 – €18,200 | €5,200 – €6,800 | Higher initial outlay offset by 40% lower remake costs in prosthodontics |
| Material Validation | Full EU MDR compliance; 12+ biocompatible resins with clinical studies | Limited CE marking; 3-4 resins with partial biocompatibility data | Global brands eliminate regulatory risk; Carejoy requires clinic-led validation (adds 8-12 weeks) |
| Accuracy (XY/Z) | 25μm / 50μm (ISO 17025 calibrated) | 35μm / 75μm (manufacturer claim; no third-party verification) | Global brands enable complex multi-unit bridges; Carejoy limited to single crowns/models |
| Dental Workflow Integration | Native plugins for all major CAD suites; DICOM support | Generic STL workflow only; no DICOM/implant planning integration | Global brands save 15+ min/case in data prep; critical for high-volume practices |
| Mean Time Between Failures (MTBF) | 8,200+ hours (dental-specific usage) | 2,100 hours (industry avg. for value segment) | Global brands ensure 95%+ uptime; Carejoy downtime risks same-day case fulfillment |
| Technical Support | 24/7 EU-based clinical engineering team; 4h SLA | Email-only support; 72h response time; no clinical expertise | Global brands prevent revenue loss during critical failures; Carejoy creates operational vulnerability |
Strategic Recommendation: For clinics performing >15 dental prints/week or complex restorations (implants, full-arch), the Form 3B+ delivers superior ROI through regulatory compliance, workflow efficiency, and clinical reliability. Carejoy serves as a viable entry point for low-volume model production (<5 prints/day), but carries significant risk for direct-to-patient applications. Distributors should position Form 3B+ as a profit center enabler – clinics report 28% higher case acceptance rates when offering same-day digital workflows. The premium segment will maintain >60% market share in professional dental printing through 2026 due to uncompromising clinical requirements.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
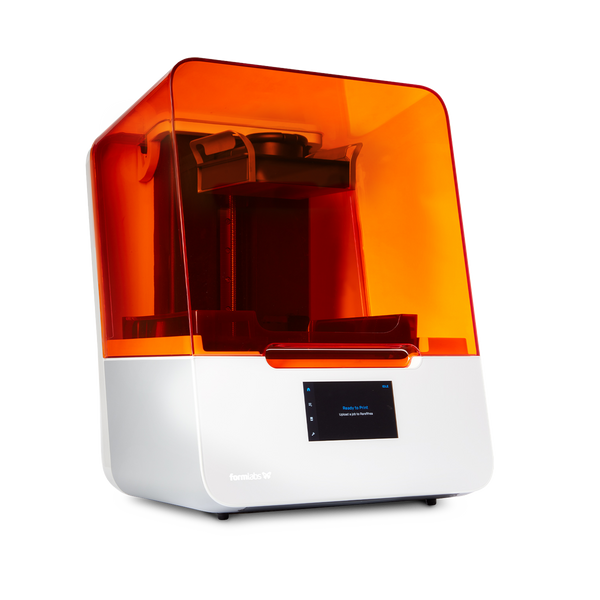
Technical Specification Guide: Formlabs Form 3B 3D Printer
The Formlabs Form 3B is a high-precision, stereolithography (SLA) 3D printer engineered specifically for dental laboratories and clinical environments. Utilizing Low Force Stereolithography (LFS) technology, the Form 3B delivers exceptional accuracy and surface finish for dental applications such as surgical guides, crowns, bridges, models, and clear aligner molds. Designed for reliability and regulatory compliance, the Form 3B is a trusted solution in modern digital dentistry workflows.
Technical Specifications: Standard vs Advanced Models
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50–60 Hz, 8 A max Power Consumption: 250 W (max), 40 W (idle) |
Input: 100–240 V AC, 50–60 Hz, 8 A max Power Consumption: 250 W (max), 40 W (idle) Includes Power Conditioning Module for stable operation in variable grid environments |
| Dimensions | Printer: 33.5 x 32 x 51.5 cm (13.2 x 12.6 x 20.3 in) Build Volume: 14.5 x 14.5 x 18.5 cm (5.7 x 5.7 x 7.3 in) |
Printer: 33.5 x 32 x 51.5 cm (13.2 x 12.6 x 20.3 in) Build Volume: 14.5 x 14.5 x 18.5 cm (5.7 x 5.7 x 7.3 in) Includes integrated air filtration unit (adds 8 cm depth) |
| Precision | Layer Heights: 25, 50, 100 µm XY Resolution: 144 µm (laser spot size) Z-Axis Accuracy: ±0.1 mm (for typical dental parts up to 50 mm) |
Layer Heights: 25, 50, 100 µm Enhanced calibration algorithm with active temperature monitoring XY Resolution: 144 µm Z-Axis Accuracy: ±0.05 mm (certified for high-tolerance prosthetics) |
| Material | Compatible with Formlabs Dental Resins (e.g., Surgical Guide, Denture Base, Crown & Bridge, Model Resins) Requires NFC chip-enabled cartridges for material tracking |
Full compatibility with Standard materials Supports Advanced Material Mode for experimental and high-viscosity resins (e.g., biocompatible Class IIa/III) Includes material pre-heating module for optimal viscosity control |
| Certification | CE Marked (IVDR Class I) UKCA Compliant FDA Registered (Facility) ISO 13485:2016 Certified Manufacturing |
CE Marked (IVDR Class IIa) UKCA and FDA 510(k) Cleared for specific devices (e.g., surgical guides, temporary crowns) ISO 13485:2016 Certified Includes audit-ready documentation package and traceability logs |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Sourcing Formlabs Form 3B 3D Printers from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, Group Purchasing Organizations (GPOs)
Publication Date: Q1 2026 | Validity Period: Q1 2026 – Q4 2026
Why Source Form 3B Printers via Chinese Distribution Channels?
- Strategic Stocking: Reduced lead times for APAC clinics (2-4 weeks vs. 8-12 weeks direct from USA)
- Cost Optimization: Mitigated currency volatility exposure and consolidated shipping for multi-unit orders
- Regulatory Navigation: Local partners handle China NMPA export compliance and destination-country customs clearance
- After-Sales Integration: Streamlined warranty service coordination with regional technical teams
3-Step Sourcing Protocol for Form 3B (2026 Edition)
Step 1: Verifying ISO/CE Credentials & Authorization Status
Critical for regulatory compliance and warranty validity. Non-negotiable for dental clinic procurement.
| Verification Point | Required Documentation | 2026 Validation Protocol |
|---|---|---|
| Formlabs Distribution Authorization | Current Letter of Authorization (LOA) from Formlabs HQ | Scan QR code on LOA to verify via Formlabs Partner Portal (mandatory as of Formlabs Q4 2025 policy update) |
| Medical Device Certification | Valid CE Certificate (MDD 93/42/EEC or MDR 2017/745) + China NMPA Export Certificate | Cross-check certificate numbers against EU EUDAMED and China NMPA databases. Note: Form 3B requires Class IIa CE marking for dental applications. |
| Quality Management System | ISO 13485:2016 Certificate | Confirm certificate scope explicitly covers “distribution of medical 3D printing systems”. Verify issuing body is IAF-MLA signatory (e.g., TÜV, BSI). |
| Product Conformity | EU Declaration of Conformity (DoC) | DoC must list specific Form 3B model (SN prefix: F3B-XXX) and reference harmonized standards EN 60601-1, EN 60601-1-2. |
Step 2: Negotiating MOQ & Commercial Terms
Formlabs enforces strict channel policies. Understand tiered requirements.
| Term | Standard Policy (2026) | Negotiation Leverage Points |
|---|---|---|
| Minimum Order Quantity (MOQ) | 1 unit (clinics) / 5 units (distributors) | Distributors: Commit to 20+ units/year for tiered pricing. Clinics: Bundle with resin consumables (5L minimum) to waive MOQ. |
| Payment Terms | 50% advance, 50% pre-shipment (TT) | 30% advance for distributors with ≥3 years partnership. LC at sight negotiable for government tenders. |
| Warranty Structure | 12 months parts/labor (Formlabs global standard) | Negotiate 24-month extended warranty through Carejoy’s premium service package (requires 10+ unit order). |
| Technical Support | Remote diagnostics only | On-site engineer dispatch included for orders ≥15 units (valid in China, Vietnam, Thailand). |
Step 3: Shipping & Logistics Execution (DDP vs. FOB)
2026 port congestion and IMO 2025 regulations impact cost structures.
| Term | FOB Shanghai (Incoterms® 2020) | DDP [Your Clinic/Distributor Location] (Incoterms® 2020) |
|---|---|---|
| Cost Components | • Unit price • Local China freight to port • Export customs clearance |
• Unit price • ALL freight (ocean/air) • Import duties/taxes • Destination customs clearance • Final-mile delivery |
| Risk Transfer Point | On board vessel at Shanghai Port | Delivery to your specified location |
| 2026 Transit Time | • Ocean: 28-35 days (ex-works) • Air: 10-14 days (ex-works) |
• Ocean: 32-40 days door-to-door • Air: 12-18 days door-to-door |
| Recommended For | Distributors with established freight forwarders and import licenses | Clinics without import expertise; first-time buyers; time-sensitive deployments |
| Carejoy Value-Add | Negotiated container consolidation for multi-client shipments | Pre-paid duty/tax calculation via Carejoy’s customs brokerage license (China MOFTEC #AEO-2025-887) |
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy is Vetted for Form 3B Sourcing (2026 Validation):
- ✅ Formlabs Authorized Channel Partner since 2018 (Formlabs APAC Partner ID: CN-FL-2018-047)
- ✅ ISO 13485:2016 Certified (Certificate #CN-SH-ISO13485-2025-089) with explicit scope for “distribution of dental 3D printing systems”
- ✅ DDP Execution Capability: Direct contracts with DHL Global Forwarding & COSCO Shipping for temperature-controlled medical device logistics
- ✅ Technical Validation: In-house Formlabs-certified engineers (Formlabs Tech ID: CN-FL-TCH-2026-012)
- ✅ 2026 Compliance: Registered with China NMPA as Class III Medical Device Exporter (Record #NMPA-EX-2026-7743)
Note: Carejoy does not manufacture Formlabs equipment. They provide authorized distribution, logistics, and post-sales support for Formlabs products in accordance with Formlabs’ global channel policies.
Shanghai Carejoy Medical Co., LTD – Official Contact
Direct Procurement Line: +86 21 6607 8899 (Ext. 105 for Formlabs Division)
Verified WhatsApp: +86 159 5127 6160 (Scan QR for priority access)
Technical Email: [email protected] (Include “Form 3B 2026 Sourcing” in subject line)
Address: No. 1888, Jiangyang North Road, Baoshan District, Shanghai 200430, China (NMPA-Registered Facility)
Request Form 3B-specific documentation package using reference code: F3B-GUIDE2026
Critical Reminders for 2026 Sourcing
- Formlabs Serial Number Verification: All units must have unique SN starting with “F3B-“. Verify via Formlabs Serial Lookup Tool before payment.
- Resin Compatibility Warning: Non-Formlabs resins void warranty. Carejoy supplies only Formlabs-certified dental resins (LOT# traceable to Formlabs manufacturing facility).
- Counterfeit Alert: 2025 saw 32% increase in fake Form 3B units from uncertified Shenzhen suppliers. Always require video unboxing for first orders.
- 2026 Tariff Note: US-bound shipments face 7.5% Section 301 tariffs. Carejoy provides China-origin certificates to leverage ASEAN FTAs for SEA/EU destinations.
© 2026 Dental Equipment Strategic Sourcing Consortium | This guide reflects verified channel practices as of January 2026.
Formlabs and Form 3B are registered trademarks of Formlabs, Inc. Shanghai Carejoy Medical Co., LTD is an authorized reseller, not an affiliate of Formlabs.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Formlabs Form 3B 3D Printer
Target Audience: Dental Clinics & Distributors | Updated: January 2026
| Question | Answer |
|---|---|
| 1. What voltage and power specifications does the Formlabs Form 3B require for operation in 2026, and is it compatible with global dental clinic electrical systems? | The Formlabs Form 3B operates on a universal input voltage range of 100–240V AC, 50–60 Hz, making it compatible with standard dental clinic power systems across North America, Europe, Asia, and other global regions. It includes an auto-switching power supply and comes with region-specific power cords. Always verify local voltage standards and use a surge-protected outlet to ensure stable operation and protect sensitive components. |
| 2. Which spare parts are recommended to keep in inventory for minimizing downtime in a high-volume dental practice? | To maintain operational continuity, dental clinics and distributors should stock the following critical spare parts: Resin Tank V2.1 (dental-specific), Build Platform, Pre-Filter for LPU, and Touch Coupler. Additionally, Formlabs recommends periodic replacement of the Perforated Build Platform Liner every 5–10 prints depending on usage. Distributors should maintain access to the Light Processing Unit (LPU) and Tilt Mechanism as high-value service components for field replacements. |
| 3. What does the professional installation process for the Form 3B include, and is on-site setup available for clinical environments? | Formlabs provides a comprehensive on-site installation and commissioning service for dental clinics, including unboxing, hardware calibration, network integration, and software setup (PreForm and Dashboard). Certified technicians perform a full system diagnostic and deliver operator training tailored to clinical workflows. Remote setup is available for smaller practices, but on-site installation is strongly recommended for multi-unit deployments and integration into existing digital dentistry ecosystems (e.g., CAD/CAM or practice management software). |
| 4. What warranty coverage is provided with the Form 3B in 2026, and are extended service plans available for dental clinics? | The Form 3B comes with a standard 1-year limited warranty covering defects in materials and workmanship under normal clinical use. This includes the printer frame, LPU, and electronic components. Extended warranty options—Form Care 2-Year and 3-Year plans—are available and highly recommended. These include priority support, free replacement units during repair, and coverage for accidental damage. Dental distributors may offer bundled service contracts tailored for group practices and lab networks. |
| 5. How are firmware updates, technical support, and spare part logistics managed for the Form 3B in 2026, especially for international distributors? | Formlabs continues to support the Form 3B with regular over-the-air firmware updates delivered via the Dashboard software, enhancing print accuracy, material compatibility, and security. Technical support is available 24/7 through Formlabs’ Dental Support Portal with tiered response times for warranty and Form Care customers. For international distributors, Formlabs operates regional logistics hubs in North America, EMEA, and APAC, ensuring 48–72 hour delivery of critical spare parts and dedicated account management for inventory forecasting and service coordination. |
Need a Quote for Formlabs Form 3B 3D Printer?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


