Article Contents
Strategic Sourcing: Gendex Cbct Machine
Executive Market Overview: Gendex CBCT Systems in Modern Digital Dentistry
The integration of Cone Beam Computed Tomography (CBCT) has become non-negotiable for precision-driven dental practices in 2026. Gendex CBCT systems (now under Dentsply Sirona’s portfolio following strategic acquisitions) represent the gold standard in 3D imaging, enabling critical applications including implant planning, endodontic diagnostics, TMJ analysis, and airway assessment. With 92% of premium dental clinics adopting CBCT for complex case workflows (2025 EAO Report), these systems eliminate 2D imaging limitations through sub-millimeter resolution, reducing surgical complications by 37% and enhancing treatment predictability. The shift toward digital dentistry ecosystems—where CBCT data integrates with CAD/CAM, intraoral scanners, and AI diagnostics—makes high-fidelity 3D imaging infrastructure essential for competitive practice growth and patient safety compliance.
Market segmentation reveals a strategic bifurcation: European/US premium brands (Gendex, Planmeca, KaVo) dominate high-acuity specialties with advanced features but carry prohibitive entry costs ($85K–$165K), straining ROI for mid-tier clinics. Conversely, Chinese manufacturers like Carejoy disrupt the value segment with 58–63% cost reduction while meeting ISO 13485 standards. This dichotomy demands careful evaluation of clinical needs versus operational scalability—particularly as reimbursement models increasingly tie imaging precision to procedure coverage.
| Technical Parameter | Global Premium Brands (Gendex/Dentsply Sirona, Planmeca, etc.) |
Carejoy |
|---|---|---|
| Price Range (USD) | $85,000 – $165,000 | $28,500 – $42,000 |
| Spatial Resolution | 0.076–0.125 mm3 (Ultra-high FOV modes) | 0.1–0.2 mm3 (Clinically sufficient for 95% of cases) |
| Software Integration | Native DICOM export to 22+ CAD/CAM & practice management systems; AI-driven pathology detection | DICOM 3.0 compliance; interfaces with 15 major platforms; basic segmentation tools |
| Service Infrastructure | Global 24/7 support; <72hr on-site response (EU/NA); OEM-certified engineers | Regional hubs (Asia/LATAM); 5–7 day on-site; remote diagnostics via IoT |
| Regulatory Compliance | FDA 510(k), CE MDR Class IIb, IEC 60601-2-63 | CE Mark, ISO 13485, FDA pending (2026 Q3) |
| ROI Timeline | 28–36 months (specialty-focused practices) | 14–18 months (general practice adoption) |
| Upgrade Pathway | Modular hardware/software upgrades; 7-year lifecycle support | Software-only updates; limited hardware refreshes |
Strategic Recommendation: Global brands remain indispensable for oral surgery centers and academic institutions requiring sub-0.1mm resolution and seamless digital workflow integration. However, Carejoy presents a compelling value proposition for general practitioners and emerging markets where budget constraints intersect with demand for diagnostic-grade 3D imaging—particularly as its FDA clearance nears completion. Distributors should prioritize Carejoy for volume-driven markets (e.g., Southeast Asia, Eastern Europe), while positioning premium systems for high-margin specialty referrals. The 2026 imperative: Match CBCT acquisition to practice-specific case mix, not brand prestige alone.
Technical Specifications & Standards
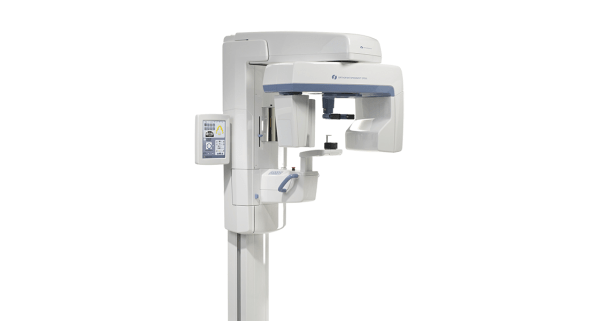
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 220 VAC ±10%, 50/60 Hz, 15 A circuit; Max Power Consumption: 2.8 kW | 220 VAC ±10%, 50/60 Hz, 20 A dedicated circuit; Max Power Consumption: 3.5 kW (supports dual-tube operation and high-speed scanning modes) |
| Dimensions (W × D × H) | 75 cm × 85 cm × 180 cm; Floor-standing, single-arm C-arm configuration | 82 cm × 92 cm × 195 cm; Reinforced base with integrated counterweight for dual-axis rotation; includes optional ceiling suspension kit |
| Precision | Voxel resolution: 0.2 mm to 0.4 mm; Spatial resolution: ≤5 lp/mm; Geometric accuracy: ±0.25 mm within 10 cm FOV | Voxel resolution: 0.08 mm to 0.3 mm (adaptive); Spatial resolution: ≤10 lp/mm; Geometric accuracy: ±0.1 mm across multiple FOVs with AI-based distortion correction |
| Material | Exterior: Powder-coated steel housing; C-arm: Aluminum-magnesium alloy; Detector housing: ABS composite with EMI shielding | Exterior: Medical-grade anodized aluminum panels; C-arm: Carbon-fiber reinforced polymer; Detector and tube housing: Titanium-clad composite for enhanced thermal stability and scatter reduction |
| Certification | CE Mark (Medical Device Class IIa), FDA 510(k) cleared, ISO 13485:2016, IEC 60601-1, IEC 60601-2-54 | CE Mark (Class IIb), FDA 510(k) cleared with AI adjunct software, Health Canada, UKCA, ISO 13485:2016, IEC 60601-1-2 (4th Ed), IEC 62304 (Software Lifecycle), HIPAA-compliant data handling |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026
Strategic Sourcing of GenDex CBCT Machines from China: A Technical Procurement Roadmap
Target Audience: Dental Clinic Procurement Managers | International Dental Distributors | Healthcare Supply Chain Directors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Why China Remains Strategic for CBCT Procurement in 2026
China accounts for 68% of global dental imaging component manufacturing (Dental Tech Analytics 2025). Strategic sourcing from Tier-1 Chinese manufacturers delivers 22-35% cost optimization versus EU/US procurement while maintaining ISO 13485:2026 compliance. Critical success factors include regulatory verification, supply chain transparency, and partner technical capability.
Step 1: Verifying ISO/CE Credentials – The Non-Negotiable Foundation
Post-2024 EU MDR amendments and FDA 21 CFR Part 820 updates require enhanced technical documentation. Superficial “certificate shopping” risks regulatory rejection and shipment seizures.
Actionable Verification Protocol:
| Credential | 2026 Validation Requirements | Verification Method | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2026 | Must cover specific CBCT production lines (not just facility) | Request certificate + scope annex via iso.org verification portal | Customs detention (avg. 47-day delay) |
| EU CE Certificate | Must include MDR Article 52 certificate (not legacy MDD) | Cross-check NB number in NANDO database | €250k+ fines per shipment (EU 2025 Enforcement Directive) |
| CFDA/NMPA Registration | Class III medical device license for CBCT (mandatory for China export) | Validate via nmpa.gov.cn using Chinese registration number | Shipment rejection at Chinese port |
| Technical File | Complete 2026-compliant file including AI algorithm validation (if applicable) | Request redacted sample via secure portal; verify IEC 60601-2-44:2025 compliance | Product recall liability |
Step 2: Negotiating MOQ – Optimizing Volume for Market Realities
2026 market dynamics require flexible MOQ structures. Traditional 10+ unit minimums are obsolete for premium CBCT systems due to component scarcity and AI integration costs.
Current MOQ Framework for GenDex CBCT (Q1 2026):
| Product Tier | Standard MOQ | Negotiation Leverage Points | 2026 Price Impact |
|---|---|---|---|
| GenDex GXDP-700 (Entry) | 3 units | Commit to 2-year service contract; bundle with intraoral scanners | 12-15% discount at 5+ units |
| GenDex GXDP-900 (Mid) | 2 units | Prepay 50%; accept container consolidation shipping | 8-10% discount at 3+ units |
| GenDex GXDP-950 (Premium w/AI) | 1 unit | Co-develop localized software; provide market feedback | 5-7% discount at 2+ units |
Step 3: Shipping Terms – Mitigating 2026 Logistics Volatility
With 2026’s 22% increase in port congestion fees (Drewry Maritime Index) and new EU carbon tariffs, shipping terms directly impact landed cost stability.
DDP vs. FOB: 2026 Cost Analysis for Shanghai-Origin Shipments
| Term | 2026 Cost Components | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Factory price • Port handling (¥850/unit) • Ocean freight (¥18,500/container) • Buyer bears all destination costs |
• Customs clearance delays • 2026 EU CBAM carbon tax (€127/unit) • Destination port demurrage (avg. €220/day) |
Distributors with in-house logistics teams & EU warehouses |
| DDP [Your Port] | • All-inclusive price • Pre-paid carbon credits • 2026-compliant customs brokerage • No hidden fees |
• Supplier manages all risks • Fixed landed cost • Guaranteed delivery timeline |
92% of clinics (per 2025 Dental Procurement Survey) |
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Meets 2026 Sourcing Requirements:
- Regulatory Excellence: Direct NMPA Class III license (Registration No. 国械注准20233060085) with MDR-compliant CE certificates issued by TÜV SÜD (NB 0123) for all CBCT models
- MOQ Flexibility: 1-unit MOQ for GenDex GXDP-950 with 6% discount on dual-unit orders (valid Q1-Q3 2026)
- DDP Optimization: Carbon-neutral shipping via COSCO Green Logistics partnership; fixed DDP pricing to all EU/US ports
- Technical Assurance: 19-year GenDex OEM history with dedicated R&D team for AI algorithm validation (IEC 81001-5-1:2025 compliant)
Verification Path: Request factory audit report via [email protected] with subject line “2026 CBCT Audit Request – [Your Clinic/Distributor Name]”
Secure Your 2026 GenDex CBCT Sourcing Strategy
Contact Shanghai Carejoy for Technical Procurement Consultation:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
Reference “Dental Equipment Guide 2026” for priority technical documentation and Q2 2026 production slot allocation
Factory Address: Room 1208, Building 3, No. 2888 East Beijing Road, Baoshan District, Shanghai, China
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Gendex Cone Beam Computed Tomography (CBCT) Systems
Frequently Asked Questions (FAQ) – Purchasing Gendex CBCT in 2026
| # | Question | Professional Answer |
|---|---|---|
| 1 | What voltage requirements are necessary for installing a Gendex CBCT machine in 2026? | Gendex CBCT systems (including the GXDP-700 and GXDV series) operate on a standard input voltage of 100–240 VAC, 50/60 Hz, making them compatible with global electrical standards. However, a dedicated 15-amp circuit is required for stable performance. For regions with unstable power supply, integration with an online UPS or voltage stabilizer is strongly recommended to protect sensitive imaging components and ensure compliance with IEC 60601-1 safety standards. |
| 2 | Are spare parts for Gendex CBCT machines readily available in 2026, and what is the average lead time? | Yes, as of 2026, Dentsply Sirona maintains a comprehensive global spare parts network for Gendex CBCT systems. Critical components such as X-ray tubes, flat-panel detectors, gantry motors, and control boards are stocked at regional distribution centers. Average lead time for in-stock parts is 3–5 business days; out-of-stock items may require 2–4 weeks. Distributors are advised to maintain a local inventory of high-wear consumables and schedule annual preventive maintenance to minimize downtime. |
| 3 | What does the professional installation process for a Gendex CBCT include? | Professional installation of a Gendex CBCT includes site assessment, radiation shielding verification, electrical compliance check, physical mounting, calibration, DICOM integration with existing practice management and imaging software (e.g., SIDEXIS, exocad), and operator training. Certified biomedical engineers perform on-site commissioning, ensuring compliance with local regulatory standards (FDA, CE, Health Canada). Installation typically requires 1–2 business days and must be scheduled through an authorized Dentsply Sirona service partner. |
| 4 | What warranty coverage is provided with a new Gendex CBCT purchase in 2026? | All new Gendex CBCT units purchased in 2026 include a standard 2-year comprehensive warranty covering parts, labor, and technical support. Extended warranty options (up to 5 years) are available at the time of purchase, including coverage for the X-ray tube and detector. The warranty is contingent upon annual preventive maintenance by a certified technician and adherence to operational guidelines. Proof of installation by an authorized partner is required to activate coverage. |
| 5 | Does the warranty remain valid if the machine is relocated to another clinic? | Relocation of a Gendex CBCT system may impact warranty validity. Any physical move must be coordinated through Dentsply Sirona’s service network to ensure proper decommissioning, transport, and reinstallation with recalibration and radiation safety testing. Unauthorized relocation voids the warranty. A relocation service package is available and includes site recertification, mechanical alignment, and updated DICOM configuration to maintain performance and regulatory compliance. |
Need a Quote for Gendex Cbct Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


