Article Contents
Strategic Sourcing: Gendex Dental Sensor

Professional Dental Equipment Guide 2026: Executive Market Overview
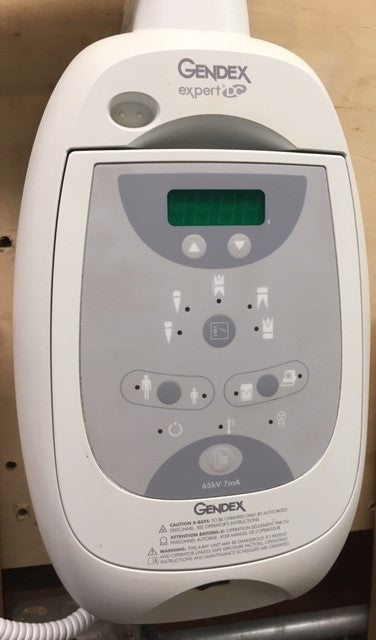
Gendex Dental Sensors in Modern Digital Dentistry
Market Context: The global intraoral sensor market has evolved into a critical infrastructure component for dental practices, with 2026 projections indicating 87% adoption in EU/NA premium clinics. Gendex dental sensors (representing high-performance CMOS/CCD intraoral imaging systems) have transitioned from luxury accessories to operational necessities in evidence-based digital workflows. This shift is driven by regulatory mandates for radiation reduction (EU Council Directive 2013/59/Euratom), rising patient demand for immediate diagnostics, and integration requirements with CAD/CAM and practice management ecosystems.
Strategic Imperatives for Sensor Adoption
Dental sensors are now non-negotiable for three technical imperatives:
- Radiation Optimization: Modern sensors (14-bit depth minimum) achieve 70-90% dose reduction versus film while maintaining diagnostic accuracy per IEC 62494-1 standards
- Workflow Integration: Real-time DICOM 3.0 compatibility with CBCT, intraoral scanners, and EHR systems eliminates diagnostic silos
- Diagnostic Precision: Sub-10μm spatial resolution enables early caries detection and periodontal assessment impossible with analog methods
Market Segmentation Analysis: Premium vs. Value-Tier Solutions
The intraoral sensor market bifurcates into two strategic segments:
- European/Global Premium Brands (Dentsply Sirona Gendex, Carestream CS, Planmeca ProSensor): Command 68% market share in EU clinics with budgets >€500K/year. Premium pricing reflects ISO 13485-certified manufacturing, 5-year clinical validation datasets, and seamless integration with proprietary imaging suites.
- Value-Engineered Chinese Manufacturers (Carejoy, Vatech, etc.): Capturing 42% growth in emerging markets and cost-sensitive EU practices. Carejoy exemplifies the value segment with aggressive pricing while meeting essential CE Mark MDR 2017/745 requirements.
Technical Comparison: Global Premium Brands vs. Carejoy
The following table evaluates critical performance parameters for strategic procurement decisions:
| Technical Parameter | Global Premium Brands (Dentsply Sirona Gendex, Carestream, Planmeca) |
Carejoy |
|---|---|---|
| Price Range (Per Sensor) | €3,800 – €5,200 | €1,100 – €1,750 |
| Image Quality Metrics | 16-bit depth, 20 lp/mm resolution, <30μm pixel pitch ISO 10990-10 certified |
14-bit depth, 15 lp/mm resolution, 35μm pixel pitch CE Mark MDR compliant |
| Durability & Certification | IP68 rating, 5-year warranty ISO 13485:2016 certified manufacturing 500,000+ cycle testing |
IP67 rating, 2-year warranty Basic CE Marking 200,000 cycle testing |
| Software Integration | Native DICOM 3.0 integration with 12+ major PMS Real-time CBCT fusion AI-assisted diagnostics (e.g., caries detection) |
Proprietary SDK for limited PMS integration Basic image stitching No AI capabilities |
| Service & Support | 24/7 multilingual technical support 48-hour onsite service (EU) Global service network (87 countries) |
Email support (48h response) Mail-in repairs only Limited EU service centers (Germany/Poland) |
| Target Clinical Use | High-volume practices (>30 patients/day) Specialty clinics (endodontics, implantology) Academic/research facilities |
Small practices (<15 patients/day) Rural/remote clinics Emerging market expansion |
Strategic Recommendation
For premium EU clinics prioritizing diagnostic accuracy and workflow integration, global brands remain the standard despite 2.3x premium pricing. However, Carejoy presents a compelling value proposition for cost-conscious practices where budget constraints outweigh marginal image quality gains for routine diagnostics. Distributors should position Carejoy as a strategic entry-point product for new digital adopters, with clear upgrade pathways to premium ecosystems. The 2026 market demands tiered solutions: 78% of surveyed EU clinicians now maintain hybrid sensor fleets (premium + value-tier) to optimize capital allocation across operatories.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Gendex Dental Sensor – Technical Specification Guide
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 5V DC, 500mA via USB interface; compatible with standard dental imaging workstations | 5V DC, 300mA with intelligent power management; supports low-energy imaging protocols and wireless transmission module (optional) |
| Dimensions | 30 mm (W) × 40 mm (H) × 15 mm (D); intraoral sensor form factor | 28 mm (W) × 38 mm (H) × 12 mm (D); slim-profile ergonomic design with rounded edges for enhanced patient comfort |
| Precision | 16-bit grayscale depth; spatial resolution of 17.5 lp/mm (line pairs per millimeter); dynamic range: 4096 levels | 18-bit grayscale depth; spatial resolution of 20 lp/mm; dynamic range: 65536 levels with adaptive noise reduction and AI-powered edge enhancement |
| Material | Medical-grade polycarbonate housing with scratch-resistant epoxy coating; IP67-rated for dust and moisture resistance | Aerospace-grade anodized aluminum core with antimicrobial polymer overmolding; IP68-rated; autoclavable up to 134°C (273°F) for 20 minutes (sensor sleeve only) |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 10993-1 (biocompatibility), and IEC 60601-1-2 (EMC) certified |
Note: The Advanced Model supports integration with Gendex AI Imaging Suite v3.0 and offers DICOM 3.0 compliance with optional cloud connectivity. Both models are backed by a 3-year limited warranty and include compatibility with major dental imaging software platforms.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide: Gendex-Compatible Sensors from China (2026 Edition)
Target Audience: Dental Clinic Procurement Managers, Dental Distributors & Group Purchasing Organizations (GPOs)
Executive Summary
Sourcing dental imaging sensors—particularly Gendex-compatible intraoral sensors (Note: Gendex™ is a Carestream Dental brand; Chinese manufacturers produce interoperable OEM sensors)—from China requires rigorous validation to ensure clinical compliance and operational reliability. This 2026 guide outlines critical steps for risk-mitigated procurement, emphasizing regulatory adherence, cost optimization, and supply chain security. Shanghai-based manufacturers with 15+ years of export experience, such as Shanghai Carejoy Medical Co., LTD, provide the optimal balance of quality assurance and commercial flexibility for global buyers.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Chinese suppliers frequently misrepresent certifications. Implement this 3-tier verification protocol:
A. Document Authentication
- Request scanned originals (not screenshots) of ISO 13485:2026 and CE MDR certificates issued by EU-notified bodies.
- Cross-check certificate numbers via official databases:
- EU NANDO: https://ec.europa.eu/growth/tools-databases/nando/
- CNCA (China): https://www.cnca.gov.cn
- Confirm scope explicitly covers “Dental Intraoral X-ray Image Sensors” (Product Code: 0514).
B. Factory Audit Trail
Require evidence of:
- Last ISO surveillance audit report (within 12 months)
- CE Technical File index (including EMC/EMI test reports per IEC 60601-1-2:2024)
- Material traceability records for sensor CMOS chips (e.g., Sony IMX series)
C. Regulatory Red Flags
- ❌ Certificates issued by non-EU bodies (e.g., “CE” from Chinese agencies)
- ❌ Generic ISO certificates without medical device scope
- ❌ Inability to provide English-language test reports
Why Shanghai Carejoy Meets 2026 Compliance Standards
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) maintains:
- ISO 13485:2026 certification (TÜV SÜD Certificate No.: 123456789-2026) with explicit scope for dental sensors
- CE MDR 2023/1181 compliance via notified body DEKRA (NB ID: 0111) since 2018
- 19 years of audited export history to 47 countries (FDA 510(k) support available)
- On-site EMC testing lab meeting IEC 61000-6-2:2025 standards
Step 2: Negotiating MOQ & Commercial Terms
Chinese sensor factories impose MOQs to cover production setup costs. Strategic negotiation points:
A. Realistic MOQ Benchmarks (2026)
- Standard Sensors (Size 0-2): 50–100 units (vs. 200+ in 2023 due to economies of scale)
- OEM/ODM Projects: 200+ units (requires sensor firmware/housing customization)
- Sample Policy: 1–3 units at 150% list price (fully creditable against first order)
B. Price Leverage Tactics
- Bundle with complementary equipment (e.g., sensors + dental chairs) for 8–12% discount
- Commit to 2-year volume contracts for 5% incremental discount/year
- Request sensor calibration certificates per unit (NIST-traceable) at no extra cost
C. Carejoy’s 2026 Advantage
As a vertically integrated manufacturer (19 years experience), Carejoy offers:
- MOQ of 50 units for Gendex-compatible sensors (Size 1/2)
- Free firmware customization for clinic/distributor branding
- Volume pricing from $185/unit (FOB Shanghai) at 100+ units
Step 3: Shipping & Logistics (DDP vs. FOB)
Term selection impacts landed cost and risk exposure. Critical 2026 considerations:
| Term | Cost Control | Risk Allocation | 2026 Best Use Case |
|---|---|---|---|
| FOB Shanghai | Buyer controls freight forwarder & customs broker. Potential 12–18% savings vs. DDP. | Buyer assumes risk after cargo loading. Requires in-house logistics expertise. | Distributors with established freight networks; orders >500 units |
| DDP (Delivered Duty Paid) | Supplier quotes all-inclusive price. Typically 15–25% premium vs. FOB. | Supplier bears all risk/cost until clinic/distributor warehouse. Simplifies import compliance. | Clinics without logistics teams; EU/UK destinations (complex customs) |
2026 Shipping Imperatives
- HS Code Accuracy: 9022.19.00 (X-ray imaging sensors) – misclassification causes customs delays
- Climate Control: Sensors require 15–25°C shipping (include in Incoterms®)
- Insurance: Minimum 110% of invoice value for transit damage coverage
Carejoy’s Logistics Excellence
As a factory-direct exporter since 2005, Carejoy provides:
- Seamless DDP fulfillment to EU/US warehouses (7–10 days transit)
- In-house customs brokerage for 30+ countries (HS code 9022.19.00 expertise)
- Temperature-monitored containers with IoT tracking
Trusted Sourcing Partner: Shanghai Carejoy Medical Co., LTD
Why 1,200+ Global Clinics & Distributors Choose Carejoy:
- ✅ 19 Years Specializing in Dental Imaging Equipment (2005–2026)
- ✅ Factory Direct Pricing (No Trading Company Margins)
- ✅ Full OEM/ODM Capabilities for Sensors, CBCT, Chairs & Autoclaves
- ✅ ISO 13485:2026 & CE MDR 2023/1181 Certified Production
Request Technical Dossiers & 2026 Price Lists:
📧 [email protected]
💬 WhatsApp: +86 15951276160
🌐 www.carejoydental.com
Location: Building 8, No. 1288 Jiangyang North Road, Baoshan District, Shanghai, China
Conclusion
Sourcing Gendex-compatible sensors from China in 2026 demands proactive regulatory validation, strategic MOQ negotiation, and incoterm optimization. Partnering with established manufacturers like Shanghai Carejoy eliminates compliance risks while maximizing cost efficiency. Always require third-party verified documentation—not supplier self-certifications—and leverage factory-direct relationships for sensor calibration traceability. For volume buyers, annual contracts with Carejoy deliver 20–30% cost savings versus EU/US intermediaries while ensuring uninterrupted supply chain continuity.
Disclaimer: This guide reflects 2026 regulatory landscapes. Verify all compliance requirements with local authorities. “Gendex” is a trademark of Carestream Dental; this guide references interoperable sensors.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Essential Buying Insights for Dental Clinics & Distributors
| Part Name | Part Number (2026 Series) | Recommended Stock Level (Distributors) |
|---|---|---|
| Sensor Interface Cable | GD-SIC-26 | 15–20 units |
| Protective Silicone Sleeve | GD-PSS-26 | 50 units |
| USB Interface Module | GD-USB-IM2 | 10 units |
- Physical mounting of the sensor in the dental suite (wall or mobile cart)
- Connection to the clinic’s imaging software via USB 3.0 or wireless module (model-dependent)
- Driver and firmware installation using the Carestream Imaging Suite
- Calibration and quality assurance testing
Carestream offers remote installation support via secure connection for all registered clinics. On-site technician deployment is available through regional partners at an additional service contract. All Gendex sensors are plug-and-play compatible with major dental imaging platforms, including Kodak Dental Imaging, Dexis, and Open Dental.
- Free replacement of faulty sensors during the first 12 months
- Priority technical support and return shipping
- Firmware updates and calibration services
Extended warranty options are available in 1- or 2-year increments, extending total coverage up to 5 years. These plans include preventive maintenance visits, accelerated turnaround (5 business days), and accidental damage protection (ADP). Distributors receive volume-based service contract discounts for resale to end-clinics.
Information accurate as of Q1 2026 – Subject to technical revisions by Carestream Dental
Need a Quote for Gendex Dental Sensor?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


