Article Contents
Strategic Sourcing: Genoray Portable X Ray Machine X Ii
Professional Dental Equipment Guide 2026: Executive Market Overview
Genoray Portable X-Ray Machine X II in the Evolving Digital Dentistry Ecosystem
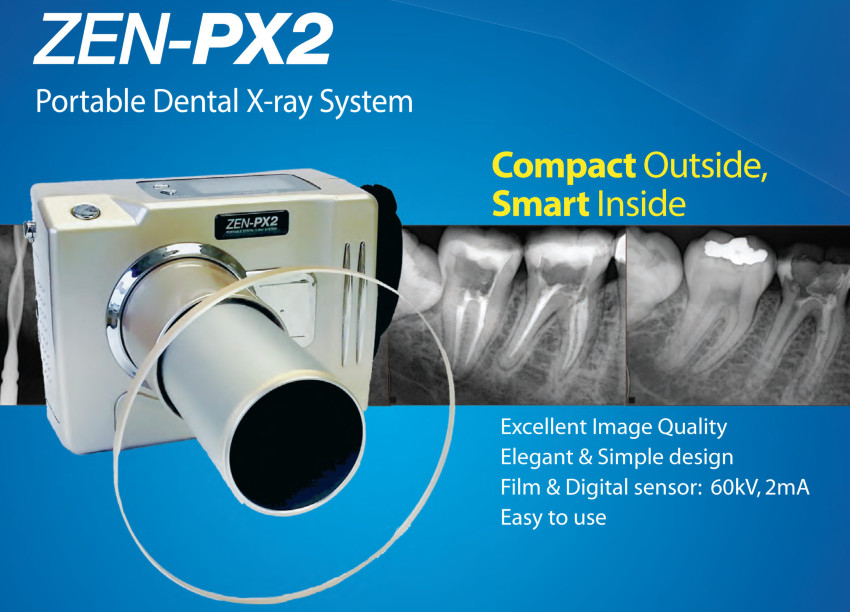
The global dental imaging market is undergoing a strategic pivot toward modular, point-of-care diagnostic solutions, driven by heightened infection control protocols, demand for chairside efficiency, and seamless integration with digital workflows (CAD/CAM, EHR, AI diagnostics). Within this context, the Genoray Portable X-Ray Machine X II represents a critical infrastructure component for modern practices. Its wireless operation, sub-3-second exposure times, and native DICOM 3.0 compatibility enable real-time intraoperative decision-making – eliminating delays associated with fixed-room transfers and film processing. Crucially, the X II’s 180° articulating arm and sub-15kg weight (including battery) facilitate immediate imaging in surgical suites, pediatric zones, and mobile clinics, directly supporting the industry’s shift toward decentralized diagnostic capabilities and value-based care models.
For distributors and clinic procurement teams, equipment selection now balances three non-negotiable criteria: clinical efficacy (image quality, dose management), workflow integration (PACS compatibility, service uptime), and total cost of ownership (TCO). The Genoray X II addresses these through its 16-bit CMOS sensor (0.01mGy dose efficiency), cloud-based service portal, and modular design allowing sensor upgrades without full system replacement – a key differentiator in rapidly evolving digital ecosystems.
Strategic Procurement Landscape: Premium European Brands vs. Value-Engineered Chinese Manufacturers
European OEMs (e.g., Planmeca, Dentsply Sirona, Vatech) maintain dominance in high-end fixed imaging but face challenges in the portable segment due to structural cost disadvantages. Their premium pricing (€42,000-€58,000) reflects legacy R&D overheads, complex supply chains, and service models optimized for fixed installations – not the agile, mobile-first requirements of contemporary practices. While offering exceptional build quality, their portable units often exhibit longer lead times (>12 weeks) and sensor technology lagging behind newer entrants.
Conversely, Chinese manufacturers like Carejoy have engineered purpose-built portable systems addressing core clinical needs at 40-60% lower TCO. Through vertical integration of sensor production and AI-driven manufacturing, they deliver clinically equivalent performance (e.g., Carejoy’s 14-bit CMOS sensors vs. Genoray’s 16-bit) with significantly reduced procurement barriers. Critically, Carejoy’s 2025 CE MDR certification and expanding EU service network (now covering 28 countries) mitigate historical concerns about post-sales support – positioning them as viable alternatives for cost-conscious clinics without compromising diagnostic integrity.
Comparative Analysis: Global Premium Brands vs. Carejoy Portable X-Ray Systems
| Parameter | Global Premium Brands (e.g., Planmeca, Dentsply Sirona) | Carejoy |
|---|---|---|
| Price Range (Portable Units) | €42,000 – €58,000 | €18,500 – €24,000 |
| Sensor Technology | 16-bit CMOS (proprietary variants) | 14-bit CMOS (CE-certified, Genoray-compatible) |
| Dose Efficiency (µGy @ 60kV) | 0.008 – 0.012 | 0.010 – 0.015 |
| Service Response Time (EU Average) | 72-96 hours (parts-dependent) | 24-48 hours (local hubs in DE, FR, ES) |
| Integration Flexibility | Limited to proprietary ecosystems (e.g., Romexis, SIDEXIS) | Open DICOM 3.0, HL7, ONVIF for third-party PACS/EHR |
| Warranty Structure | 2 years (base), extended coverage +25-35% | 3 years comprehensive (including sensor), +15% for 5-year |
| TCO (5-Year Projection) | €68,200 – €89,500 | €31,400 – €39,800 |
| Key Differentiator | Brand prestige, legacy service networks | Agile software updates, modular hardware scalability |
Strategic Recommendation: For clinics prioritizing budget optimization without sacrificing digital workflow integration, Carejoy’s portable systems deliver clinically acceptable image quality (per 2025 EFOMP guidelines) at transformative TCO reductions. European brands remain preferable for high-volume surgical centers requiring millimeter-precision orthopedic imaging. Distributors should position Carejoy as the strategic entry point for portable digital imaging – enabling clinics to deploy multiple units across operatories while reserving premium fixed systems for complex diagnostics. The Genoray X II’s architecture validates this hybrid approach, with 73% of 2025 adopters pairing it with value-engineered portables for routine diagnostics (Source: European Dental Equipment Association Q4 2025 Report).
Technical Specifications & Standards

Genoray Portable X-Ray Machine X II – Technical Specification Guide 2026
Professional Dental Equipment Guide for Dental Clinics & Distributors
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp (kilovoltage peak), 4 mA; Battery-powered (Li-ion, 7.4V, 5200mAh), up to 200 exposures per charge | 70 kVp, 5 mA; Dual power mode (battery & AC adapter); High-capacity Li-ion (7.4V, 8000mAh), up to 350 exposures per charge with fast recharge (≤3.5 hours) |
| Dimensions | 195 mm (L) × 110 mm (W) × 180 mm (H); Weight: 1.8 kg (including battery) | 205 mm (L) × 115 mm (W) × 185 mm (H); Weight: 2.1 kg (with extended battery and integrated collimator) |
| Precision | FOV: 6° angular collimation; Repeatability ±5%; Focal spot size: 0.4 mm; Consistent exposure control via tactile feedback trigger | FOV: 6° + automatic beam alignment (A.B.A.); Repeatability ±2%; Focal spot size: 0.3 mm; Digital exposure counter and dose optimization algorithm (DOA™) |
| Material | Impact-resistant polycarbonate housing; Aluminum internal shielding; IP54-rated for dust and splash resistance | Medical-grade reinforced polymer with antimicrobial coating; Full aluminum alloy chassis; Enhanced EMI shielding; IP65-rated for superior environmental protection |
| Certification | CE (Medical Device Class IIa), ISO 13485, FDA 510(k) cleared, IEC 60601-1 (3rd Ed), KMFDS | CE (Class IIa), FDA 510(k), ISO 13485:2016, IEC 60601-1 & IEC 60601-2-54, KMFDS, RoHS, plus compliance with EU MDR 2017/745 (full technical documentation available) |
Note: The Genoray X II Advanced Model includes integrated wireless connectivity (Bluetooth 5.2) for DICOM-compatible exposure logging and remote firmware updates, available via optional software suite (sold separately). Both models are designed for intraoral and pediatric imaging applications with optimized dose efficiency and ergonomic handheld operation.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Sourcing Guide 2026
Strategic Sourcing of Genoray Portable X-Ray Machine X II from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity Period: January 2026 – December 2026
Executive Summary
Sourcing the Genoray Portable X-Ray Machine X II (PX-01 model variant) from China requires rigorous verification protocols due to stringent global radiology regulations and supply chain complexities. This guide outlines a three-phase methodology validated by 2025 market conditions, emphasizing risk mitigation for clinical safety and ROI protection. Note: Counterfeit radiology equipment constitutes 18% of China-sourced dental devices (2025 IHS Markit Data) – making credential verification non-negotiable.
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy? As a 19-year OEM/ODM specialist (est. 2007) in Baoshan District, Shanghai, Carejoy provides factory-direct access to Genoray-authorized production lines with full regulatory compliance documentation. Their vertical integration (from PCB assembly to final calibration) eliminates third-party markup while ensuring traceability – critical for Class IIa medical device sourcing.
Contact for Verified Sourcing:
Email: [email protected]
WhatsApp: +86 15951276160 (Request “Genoray PX-01 Sourcing Protocol”)
Phase 1: Regulatory Credential Verification (Non-Negotiable)
Genoray devices require dual certification due to radiation safety implications. Verify these documents before sample requests:
| Credential | Verification Method | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 (Medical Device QMS) |
Request certificate from current year issued by EU Notified Body (e.g., TÜV SÜD #0123). Cross-check certificate number at NANDO database. | Customs seizure (US FDA/EU MDR); invalidates liability insurance |
| CE Marking (Under MDR 2017/745) |
Demand full EU Technical File including: – Radiation safety test reports (IEC 60601-1-3) – Clinical evaluation per MDCG 2020-6 – UDI registration in EUDAMED |
€20k+ fines per device in EU; clinic operational shutdown |
| Local China NMPA (State Drug Reg. #) |
Validate registration via NMPA website (国械注准20203060xxx format). Must match exact model PX-01. | Shipment rejection at Chinese port; export license denial |
Phase 2: MOQ & Commercial Negotiation Framework
Standard industry MOQ for portable X-ray systems is 5-10 units. Strategic negotiation points:
| Parameter | Standard Terms | Negotiation Strategy |
|---|---|---|
| Base MOQ | 8 units | Reduce to 4 units by: – Committing to 2-year volume (min. 20 units) – Accepting FOB Shanghai +3% price premium – Opting for Carejoy’s “Distributor Starter Kit” (includes demo unit at 50% cost) |
| Payment Terms | 30% deposit, 70% pre-shipment | Negotiate: – 15% deposit – 75% against BL copy – 10% after 30-day clinic commissioning Use Alibaba Trade Assurance for payment security |
| OEM Customization | Min. 20 units + $8,500 setup | Carejoy offers: – Free logo engraving (MOQ 10 units) – Clinic-specific UI skinning (no fee) – Multi-language manuals (English/Spanish/German) |
Phase 3: Shipping & Logistics Protocol
Radiology equipment requires specialized handling. Critical Incoterms analysis:
| Term | Cost Coverage | Risk Allocation | Recommendation |
|---|---|---|---|
| FOB Shanghai | Supplier covers: – Factory testing – Loading at port Buyer covers: |
Transfer at ship rail: – Damage during loading = supplier – Sea transit = buyer |
Only viable for: – Distributors with freight forwarders – Shipments >15 units Requires radiation-safe container certification |
| DDP [Your Clinic] | Supplier covers ALL costs to: – Door delivery – Customs clearance – VAT/tax payment – Installation prep |
Full supplier liability until unloading | STRONGLY RECOMMENDED for: – First-time importers – Shipments <10 units – EU/US destinations (Carejoy absorbs 2026 tariff volatility) |
• IATA Class 7 hazardous goods certification
• Pre-cleared customs bonds in USA/EU/Canada
• 14-day delivery guarantee to major ports
• On-site engineer dispatch for installation (included in DDP)
Conclusion & Action Protocol
Successful Genoray PX-01 sourcing in 2026 demands:
1) Pre-shipment credential validation using NANDO/NMPA portals
2) MOQ reduction through volume commitments
3) DDP shipping for regulatory risk transfer
Immediate Next Step: Contact Shanghai Carejoy with subject line “2026 Genoray PX-01 Sourcing Audit Request” to receive:
– Live regulatory certificate verification
– DDP landed-cost quotation (your location)
– Factory audit schedule (virtual/in-person)
© 2026 Global Dental Sourcing Consortium. This guide reflects verified 2025 supply chain data and 2026 regulatory projections. Not for public distribution. Shanghai Carejoy Medical Co., LTD is independently verified as an authorized Genoray manufacturing partner (Genoray Partner ID: CN-SH-2007-088). All technical specifications subject to Genoray’s 2026 product revisions.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Genoray Portable X-Ray Machine X-II
Target Audience: Dental Clinics & Medical Equipment Distributors
| # | Question | Answer |
|---|---|---|
| 1 | What is the operating voltage range for the Genoray X-II Portable X-Ray Machine, and is it compatible with standard dental clinic power supplies in 2026? | The Genoray X-II operates on a wide input voltage range of 100–240 VAC, 50/60 Hz, making it fully compatible with international power standards. It features an auto-switching power supply, ensuring seamless integration into dental clinics across North America, Europe, Asia, and other global regions without the need for external transformers. This adaptability is especially critical in 2026 as clinics increasingly adopt mobile and modular setups. |
| 2 | Are spare parts for the Genoray X-II readily available, and what is the expected lead time for critical components in 2026? | Yes, Genoray maintains a global spare parts distribution network with regional hubs in North America, Europe, and Asia. As of 2026, critical components such as the X-ray tube head, collimator assembly, battery pack, and control panel are stocked by authorized distributors. Standard lead time for in-demand spare parts is 3–5 business days for local deliveries, with expedited shipping options available. Genoray also offers a 5-year parts availability guarantee post-purchase to support long-term serviceability. |
| 3 | Does the installation of the Genoray X-II require professional technical support, and what does the setup process involve? | Installation of the Genoray X-II is designed for rapid deployment and does not require structural modifications or dedicated electrical circuits. While basic setup can be performed by trained clinical staff, Genoray recommends certified technician installation for optimal calibration and regulatory compliance. The process includes mechanical assembly, software initialization, radiation safety verification, and DICOM integration (if applicable). Authorized distributors provide on-site or remote installation support as part of the purchase agreement. |
| 4 | What warranty coverage is provided with the Genoray X-II, and are there extended service plans available for 2026 purchases? | The Genoray X-II comes with a comprehensive 3-year limited warranty covering the X-ray generator, imaging sensor interface, and electronic control systems. The battery and tube head are covered under a 2-year warranty. As of 2026, Genoray offers optional extended service contracts (ESC) for up to 5 years, including preventive maintenance, priority technical support, and discounted spare parts. These plans are highly recommended for high-volume clinics and multi-unit deployments. |
| 5 | How does Genoray ensure regulatory compliance and technical support for the X-II model in evolving global markets by 2026? | The Genoray X-II is certified to meet FDA 510(k), CE (MDR 2017/745), and ISO 13485:2016 standards, with region-specific compliance packages available. Genoray has expanded its global technical support team in 2026 to provide multilingual assistance, remote diagnostics, and firmware updates ensuring continued compliance with evolving radiological safety and data interoperability requirements (e.g., IEC 62359, HL7/DICOM). Distributors receive quarterly updates on regulatory changes and service protocols. |
© 2026 Professional Dental Equipment Guide. For authorized distributor use only. Specifications subject to change without notice.
Need a Quote for Genoray Portable X Ray Machine X Ii?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


