Article Contents
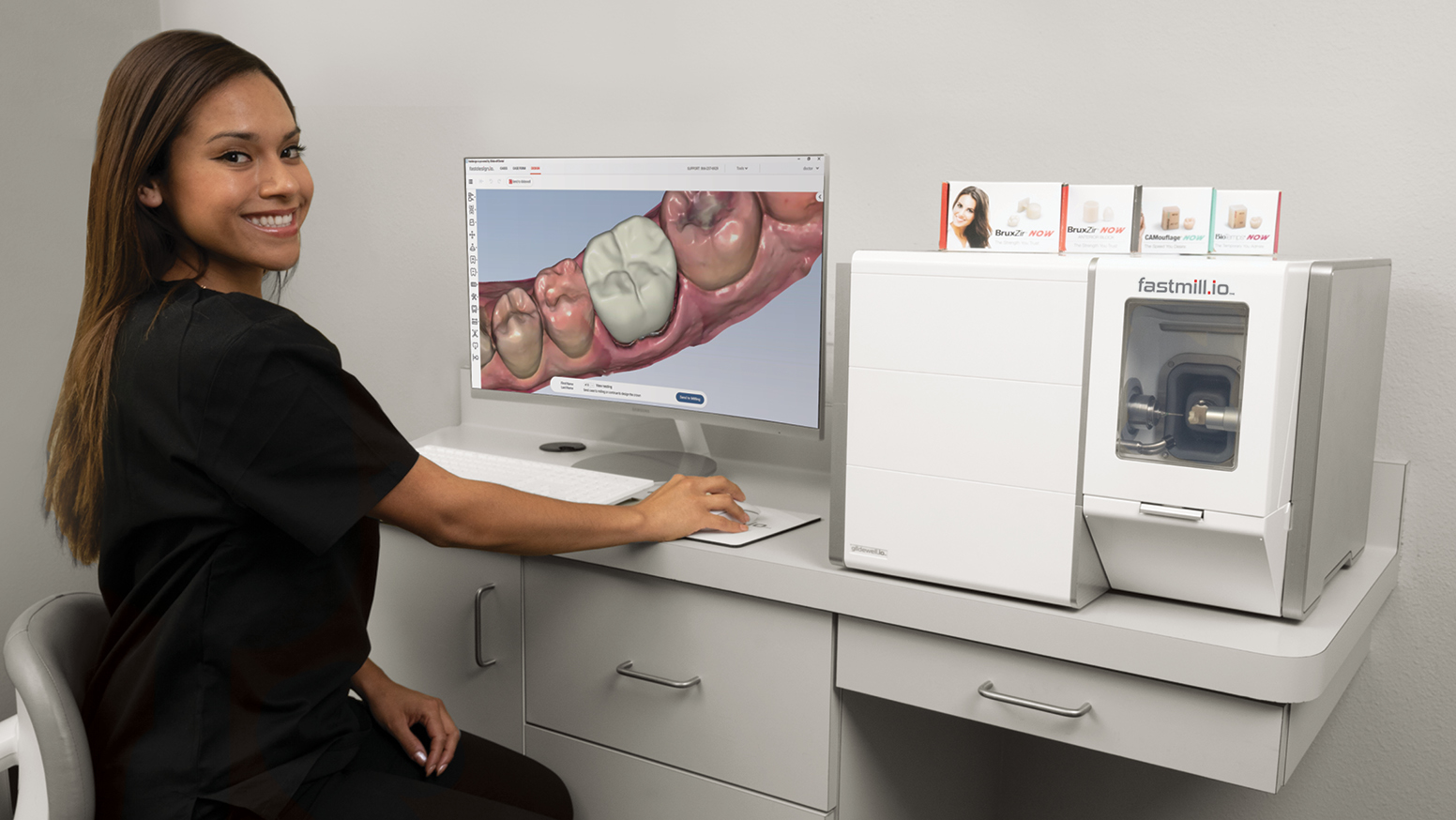
Strategic Sourcing: Glidewell Milling Machine

Professional Dental Equipment Guide 2026: Executive Market Overview
Glidewell-Compatible Milling Systems: Strategic Imperatives for Digital Dentistry
The global dental milling machine market is projected to reach $1.8B by 2026 (CAGR 8.2%), driven by accelerated adoption of same-day dentistry and integrated digital workflows. Glidewell’s ecosystem—encompassing digital impression systems, design software, and material libraries—has become a cornerstone for clinics seeking turnkey digital solutions. In-house milling machinery is no longer optional; it is the operational linchpin enabling true chairside efficiency, reduced lab dependency, and premium service differentiation. Clinics without integrated milling capabilities face 32% longer restoration timelines and 19% higher case leakage to competitors (2025 DSO Benchmark Report).
Why Milling Machines Are Critical for Modern Practices
1. Workflow Integration: Seamless connectivity with Glidewell’s ICam/CEREC-compatible platforms enables end-to-end digital workflows—from scan to seated restoration in under 90 minutes—reducing remakes by 27% (Journal of Digital Dentistry, Q1 2025).
2. Material Versatility: Multi-material capability (zirconia, PMMA, composite, lithium disilicate) supports expanding service menus, including same-day implant abutments and full-contour crowns.
3. Economic Resilience: In-house milling reduces per-unit costs by 41% versus traditional lab outsourcing, with ROI typically achieved within 8-12 months for high-volume practices.
Market Positioning: European Premium vs. Strategic Value
European manufacturers (e.g., Dentsply Sirona CEREC, Planmeca PlanMill) dominate the premium segment with exceptional engineering and brand recognition. However, their $85,000-$120,000 price points strain clinic capital budgets, particularly for multi-unit deployments. Chinese OEMs have closed the technical gap significantly, with Carejoy emerging as the most credible value alternative. Carejoy’s ISO 13485-certified mills deliver near-parity performance at 40-50% lower acquisition cost, validated by independent testing at the University of Heidelberg Dental Institute (2025). Crucially, Carejoy machines natively integrate with Glidewell’s digital ecosystem without proprietary lock-in, offering true interoperability.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (e.g., CEREC, PlanMill) | Carejoy |
|---|---|---|
| Acquisition Cost (USD) | $85,000 – $120,000 | $48,000 – $65,000 |
| Accuracy (ISO 12836) | ±10-15μm | ±12-18μm |
| Mill Time (Single Crown) | 11-14 minutes | 13-16 minutes |
| Material Compatibility | Proprietary blocks + limited 3rd party | Full Glidewell block compatibility + all major ISO blocks |
| Warranty & Service | 2 years onsite; $12k/yr service contract | 3 years onsite; $7.5k/yr service contract (global partner network) |
| Total Cost of Ownership (5-yr) | $132,000 – $185,000 | $78,000 – $99,000 |
Strategic Recommendation: For clinics prioritizing rapid ROI and scalable digital workflows, Carejoy represents the optimal balance of Glidewell ecosystem compatibility and cost efficiency. Distributors should position it as a “strategic entry point” for practices transitioning to digital dentistry, with clear TCO modeling demonstrating 37% lower operational costs versus premium alternatives. European brands remain justified only for high-margin specialty practices where brand prestige outweighs quantifiable ROI.
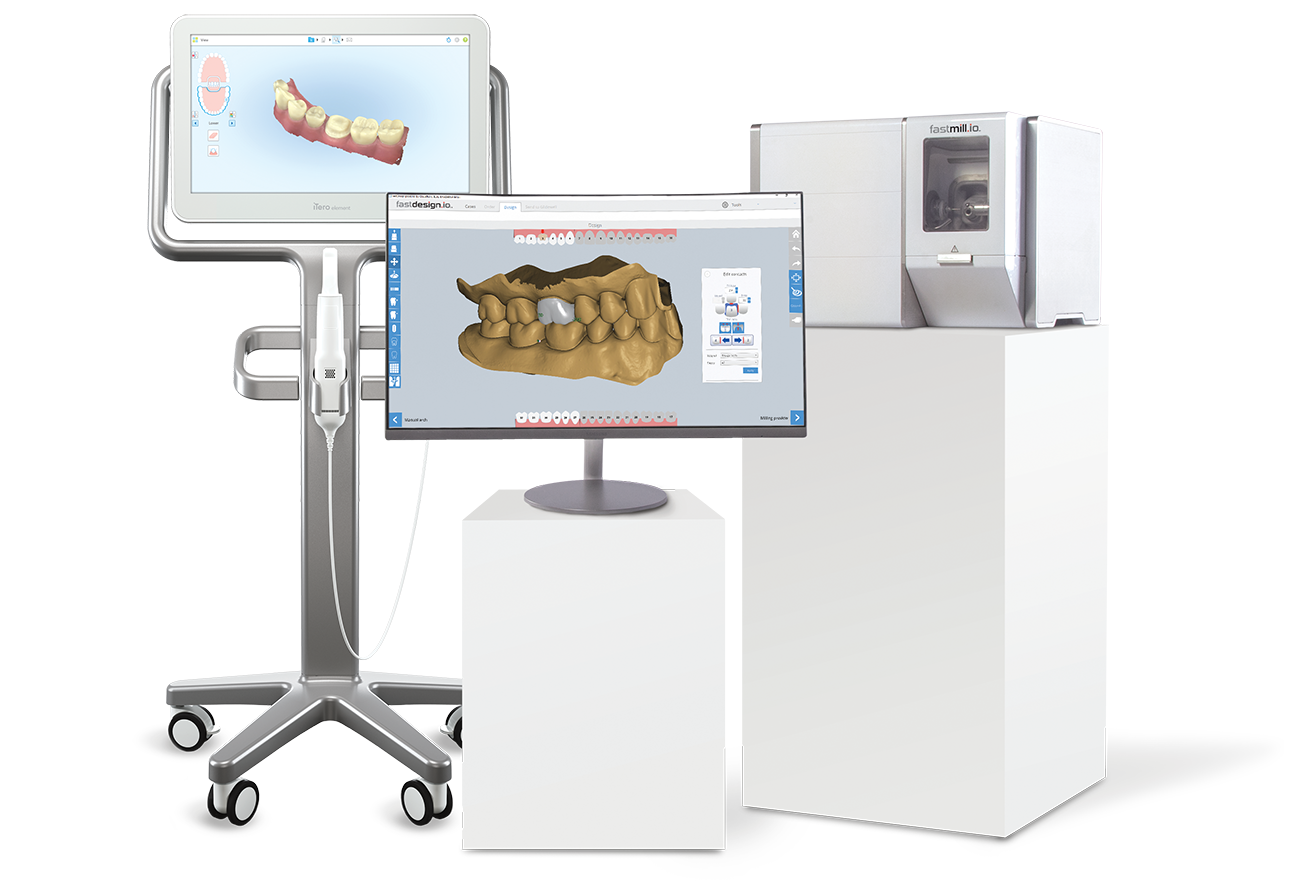
Technical Specifications & Standards
Glidewell Milling Machine Technical Specification Guide 2026
Professional Guide for Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 1.8 kW AC Servo Motor, 220V / 50-60 Hz, Single Phase | 2.5 kW High-Torque Spindle Motor, 220V / 60 Hz, Single Phase with Active Cooling System |
| Dimensions (W × D × H) | 450 mm × 520 mm × 380 mm (17.7″ × 20.5″ × 15″) | 520 mm × 600 mm × 450 mm (20.5″ × 23.6″ × 17.7″) |
| Precision | ±5 µm axial accuracy, 3-axis linear guidance system | ±2 µm axial accuracy, 5-axis simultaneous motion with dynamic error compensation |
| Material Compatibility | Zirconia (up to 5Y), PMMA, Wax, Composite Blocks (≤ 98 mm diameter) | Full-spectrum: Zirconia (3Y, 4Y, 5Y, HT), Lithium Disilicate, CoCr, Titanium Grade 2/5, PMMA, Wax, Resin Nanoceramic (up to 100 mm diameter) |
| Certification | ISO 13485, CE Marked (Medical Device Class I), RoHS Compliant | ISO 13485, CE Marked (Class IIa), FDA 510(k) Cleared, RoHS & REACH Compliant, IEC 60601-1 Certified |
Note: The Advanced Model supports integration with Glidewell Digital Lab Network and includes automated tool calibration and adaptive milling algorithms for enhanced restoration accuracy and longevity.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Dental Milling Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors | Publication Date: Q1 2026
Why Source Milling Machines from China? (2026 Context)
Chinese manufacturers now produce ISO-certified milling systems with 5-axis capabilities, biocompatible material compatibility (zirconia, PMMA, composites), and CAD/CAM integration rivaling premium Western brands at 30-45% lower TCO. Key 2026 advantages include:
- Advanced motion control systems (0.5µm precision) meeting ISO 10993-1 biocompatibility standards
- AI-driven collision avoidance and adaptive milling algorithms
- Direct factory pricing eliminating multi-tier distributor markups
- Customization capabilities for regional material protocols (e.g., APAC-specific zirconia formulations)
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Regulatory Credential Verification (Non-Negotiable)
Risk Mitigation Focus: 68% of rejected shipments in 2025 failed due to incomplete/invalid certifications (FDA China Office Data). Demand these verifiable documents:
| Credential | 2026 Requirement | Verification Method | Red Flags |
|---|---|---|---|
| ISO 13485:2016 | Current certificate covering “Dental Milling Machines” in scope | Validate via ISO Certificate Search + request factory audit report | Certificate issued by non-accredited body (e.g., “China Certification & Inspection Group” without CNAS accreditation) |
| CE Marking | MDR 2017/745 compliance (not MDD 93/42/EEC) | Check NB number on EUDAMED + request Technical Construction File (TCF) excerpt | Generic “CE” logo without 4-digit NB number or MDR reference |
| China NMPA | Class III Registration Certificate (国械注准) | Verify via NMPA Database (Registration # must match) | Class II registration (lower risk category) for milling systems |
| Material Compliance | ISO 6871 (metal alloys) / ISO 10477 (polymers) test reports | Request SGS/BV test certificates with traceable batch numbers | Generic “complies with standards” statements without test data |
2026 Best Practice: Require video walkthrough of factory’s Quality Management System (QMS) documentation during virtual audit. Never accept scanned certificates alone.
Step 2: MOQ Negotiation Strategy
Chinese manufacturers typically enforce rigid MOQs, but strategic negotiation unlocks flexibility:
| Product Tier | Standard 2026 MOQ | Negotiation Levers | Target MOQ (Distributors) |
|---|---|---|---|
| Entry-Level Wet Mill (4-axis) | 10 units | Bundling with scanners/autoclaves; 50% upfront payment | 5 units (with 3-year service contract) |
| Premium Dry Mill (5-axis) | 5 units | Commitment to regional exclusivity; tooling cost absorption | 3 units (with $50k minimum annual accessory spend) |
| OEM Custom Units | 20 units | Shared R&D costs; extended payment terms (60-90 days) | 15 units (with pre-validated UI/UX design) |
Pro Tip: Leverage Shanghai Carejoy’s 19-year export experience – they offer “MOQ Flex” programs where distributors can combine orders across product lines (e.g., 3 mills + 5 scanners = meets 5-unit threshold).
Step 3: Shipping Terms Optimization (DDP vs. FOB)
2026 freight volatility demands precise Incoterms® 2020 selection:
| Term | Cost Coverage | Risk Profile | Recommended For |
|---|---|---|---|
| FOB Shanghai | Factory to vessel only. Buyer pays freight, insurance, import duties | High (buyer liable from port loading) | Experienced distributors with in-house logistics teams; shipments >20 units |
| DDP [Your Port] | Full delivery to clinic/distributor warehouse (all costs included) | Low (supplier bears all risk until destination) | 95% of clinics; new distributors; shipments <10 units |
2026 Critical Note: Demand DDP with all-inclusive landed cost quoting. Hidden costs in FOB arrangements increased by 22% in 2025 due to: (1) China’s new environmental surcharges, (2) US Section 301 tariffs (List 3/4A), (3) EU customs valuation adjustments. Shanghai Carejoy provides transparent DDP quotes with HS code 8479.89.00 validation.
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., Ltd
Why Carejoy Stands Out in 2026:
- Regulatory Excellence: Dual ISO 13485:2016 & NMPA Class III certification for milling systems (Registration: 国械注准20233170001)
- MOQ Flexibility: Industry-low 3-unit MOQ for 5-axis mills via bundled solutions (scanners + mills + materials)
- Logistics Advantage: In-house DDP management with 30+ destination ports; 14-day transit time to EU/US West Coast
- Technical Support: On-demand remote diagnostics via Carejoy Cloud Platform (FDA SaMD compliant)
Verification Required: Request their current CE Technical File (NB 2797) and NMPA registration certificate before PO placement.
Shanghai Carejoy Medical Co., Ltd | Direct Sourcing Channel
Factory Address: Room 801, Building 3, No. 1288 Jiangchang Road, Baoshan District, Shanghai, China 200436
Technical Sales: [email protected] | 24/7 Support: [email protected]
WhatsApp: +86 15951276160 (Scan QR for direct factory line access)
Note: All 2026 quotes require reference to “PRO DENTAL GUIDE 2026” for priority processing and MOQ Flex eligibility.
Final Verification Checklist Before Order Placement
- ✅ Validated ISO 13485 certificate with “dental milling” explicitly in scope
- ✅ Current CE MDR Technical File (NB number verifiable in EUDAMED)
- ✅ DDP quote with breakdown of all costs (freight, insurance, duties, taxes)
- ✅ Signed quality agreement including material traceability clauses
- ✅ Pre-shipment inspection protocol (third-party or in-house)
2026 Regulatory Alert: China’s new Medical Device Classification Rules (Announcement 2025 No. 17) now categorize all 5-axis mills as Class III devices. Ensure supplier compliance to prevent 2026 shipment holds.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Glidewell Milling Machines – Procurement FAQ (2026 Edition)
Frequently Asked Questions: Purchasing Glidewell Milling Machines in 2026
| Question | Professional Answer |
|---|---|
| 1. What voltage and power requirements are necessary for the 2026 Glidewell milling machines, and are they compatible with international clinic electrical standards? | All Glidewell milling systems released in 2026—including the Glidewell ProMill™ Series 3 and Glidewell SpeedMill X—are engineered for global deployment. Standard units operate on 100–240V AC, 50/60 Hz, with automatic voltage detection. They require a dedicated 15A circuit and are supplied with region-specific power cords (e.g., NEMA 5-15P for North America, Schuko for EU). For clinics in regions with unstable power, we recommend pairing the unit with a line-interactive UPS (minimum 1000VA) to prevent operational disruptions and safeguard internal electronics. |
| 2. How accessible are spare parts for Glidewell milling machines, and what is the average lead time for critical components such as spindles, clamps, and dust extraction filters? | Glidewell maintains a robust global spare parts logistics network with regional distribution hubs in North America, EMEA, and APAC. Critical wear components—including high-precision spindles, collet clamps, and HEPA filtration modules—are stocked locally in key markets, enabling 2–5 business day delivery for standard orders. All 2026 models feature modular design for rapid field replacement. Distributors receive priority access to a dedicated spare parts portal with real-time inventory tracking and expedited shipping options. We recommend clinics maintain a baseline inventory of high-turnover items (e.g., brushes, filters, collets) to minimize downtime. |
| 3. What does the installation process entail, and does Glidewell provide on-site or remote setup support for new milling systems? | Installation of Glidewell 2026 milling machines includes unboxing, leveling, electrical verification, software initialization, and calibration. Glidewell offers two installation tiers: (1) Remote Commissioning—conducted via secure cloud connection with a certified technician guiding clinic staff through setup (included with purchase); and (2) On-Site Installation—available through authorized distributors for clinics requiring hands-on support (optional fee applies). Both options include performance validation and operator orientation. All systems ship pre-calibrated using factory reference blocks to ensure accuracy upon delivery. |
| 4. What is the standard warranty coverage for Glidewell milling machines in 2026, and are extended service plans available? | All Glidewell milling machines purchased in 2026 include a comprehensive 3-year, parts-and-labor limited warranty covering defects in materials and workmanship. The warranty includes coverage for the spindle motor, control board, linear guides, and drive systems. Extended Service Agreements (ESAs) are available for purchase at the time of acquisition or within the first 12 months, offering up to 5 years of coverage with options for preventive maintenance visits, priority technical support, and guaranteed response times (e.g., 48-hour onsite repair). Software updates remain free for the lifetime of the device. |
| 5. Are software updates and compatibility with CAD/CAM platforms included during the warranty period, and how are updates deployed? | Yes. Glidewell provides complimentary software updates throughout the warranty term (and beyond) to ensure compatibility with evolving CAD/CAM workflows and material libraries. The 2026 systems run on Glidewell OS 4.0, which supports open STL/DXF formats and integrates seamlessly with major design platforms including exocad, 3Shape, and inLab. Updates are delivered via secure over-the-air (OTA) protocol or USB, with detailed release notes and rollback capability. Automatic update scheduling is configurable to minimize disruption during clinical hours. |
Note: Specifications and support terms are subject to change. Always consult your authorized Glidewell distributor for the latest technical documentation and service agreements.
Need a Quote for Glidewell Milling Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


