Article Contents
Strategic Sourcing: Glidewell Milling Unit
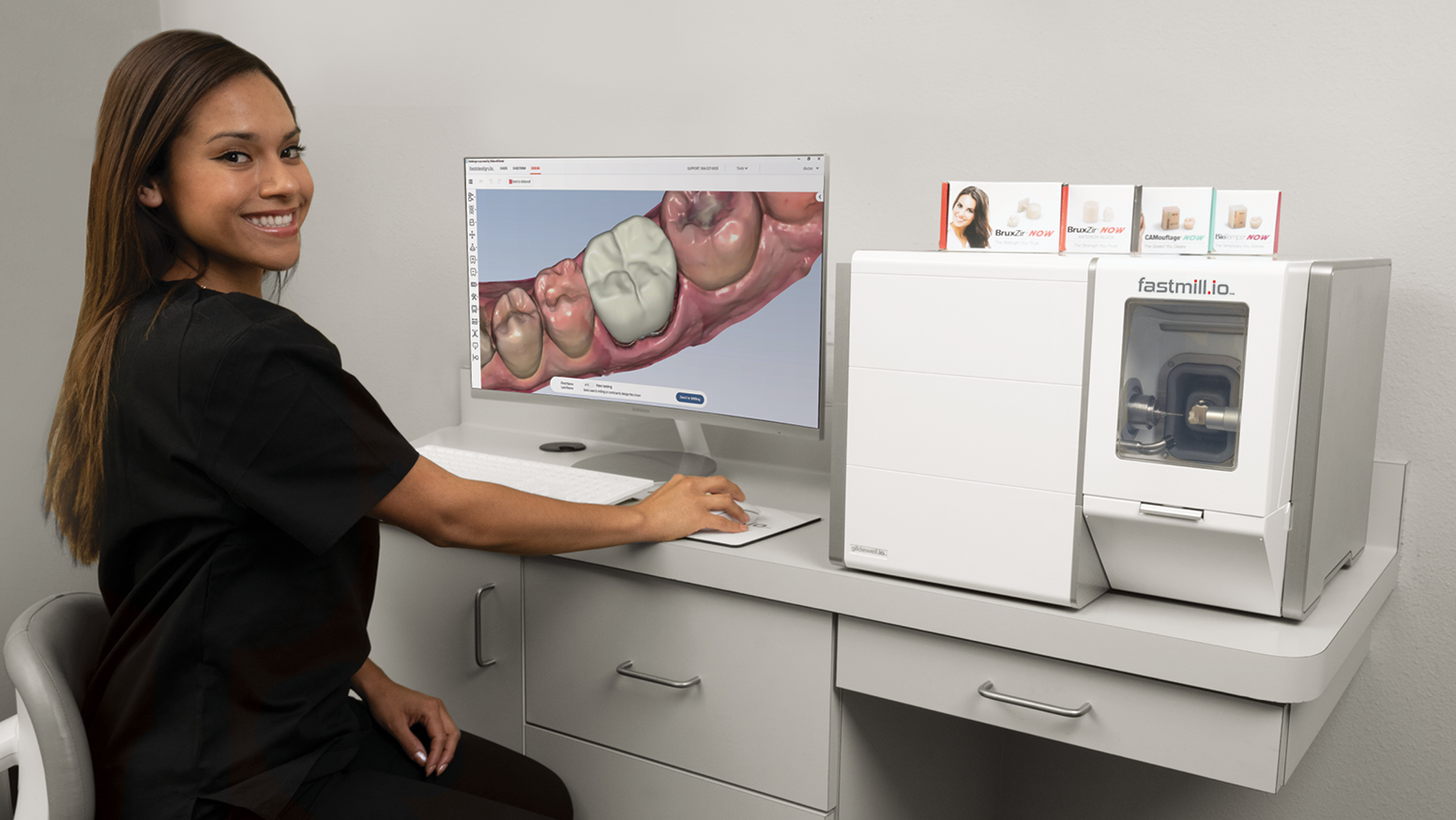
Technical Specifications & Standards

Glidewell Milling Unit Technical Specification Guide 2026
Professional Guide for Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 800 W AC Motor, 24 V DC Input | 1200 W High-Torque Brushless DC Motor, 48 V DC Input with Active Cooling |
| Dimensions (W × D × H) | 420 mm × 510 mm × 380 mm | 480 mm × 560 mm × 420 mm (Includes integrated dust extraction) |
| Precision | ±5 µm positional accuracy, 3-axis linear guidance | ±2 µm positional accuracy, 5-axis synchronized motion with dynamic error compensation |
| Material Compatibility | Zirconia (up to 4Y), PMMA, Wax, Composite Blocks (≤ 98 mm diameter) | Full-spectrum: 3Y/4Y/5Y Zirconia, Lithium Disilicate, CoCr, Titanium Grade 2/5, PMMA, Wax, Hybrid Ceramics (up to 100 mm diameter) |
| Certification | ISO 13485, CE Marked (Medical Device Directive 93/42/EEC), FCC Class A | ISO 13485:2016, CE Marked (MDR 2017/745), FDA 510(k) Cleared, IEC 60601-1, RoHS 3 Compliant |
Note: The Advanced Model supports integration with Glidewell Digital Network (GDN) for remote diagnostics, automated firmware updates, and cloud-based production tracking. Both models include IP54-rated enclosures for dust and debris resistance.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Sourcing Dental Milling Units from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Why Source Milling Units from China in 2026?
Strategic sourcing from China offers 30-45% cost savings versus Western OEMs while maintaining ISO-grade quality. Key drivers include advanced CNC manufacturing capabilities, rapid prototyping for OEM/ODM projects, and scalable production for distributors. Critical success factors: Regulatory compliance, technical validation, and logistics management.
3-Step Sourcing Protocol for Dental Milling Units
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
| Verification Point | Industry Standard (2026) | Risk of Non-Compliance | Action Required |
|---|---|---|---|
| ISO 13485:2016 Certification | Must cover design, manufacturing & servicing of dental milling units | Customs seizure (EU/US), voided clinic insurance, legal liability | Request certificate + scope page; validate via iso.org or notified body database |
| EU CE Marking | Must include MDR 2017/745 (Class IIa) with NB number | Market ban in EEA, distributor liability under Article 16 MDR | Demand full EU Declaration of Conformity + technical file index |
| US FDA Registration | Required for US-bound units (even if not 510(k) cleared) | Shipment rejection at US ports, FDA Form 482 inspection triggers | Confirm facility is listed under FDA FEI number |
Pro Tip: Reject suppliers who provide only “CE” self-declarations without notified body involvement. MDR-compliant CE requires NB oversight for Class IIa devices.
Step 2: Negotiating MOQ & Commercial Terms
| Term | Market Standard (2026) | Carejoy Advantage | Negotiation Strategy |
|---|---|---|---|
| Minimum Order Quantity (MOQ) | 5-10 units (standard for new buyers) | 1 unit for validated distributors; 3 units for clinics | Leverage sample unit approval to reduce MOQ for first order |
| Payment Terms | 30% deposit, 70% pre-shipment (T/T) | 20% deposit, 70% against BL copy, 10% after 30-day field test | Request escrow for first-time partnerships; avoid 100% upfront |
| Warranty | 12 months parts/labor (excludes consumables) | 24 months comprehensive + remote diagnostics support | Specify warranty start date as clinic installation date (not ex-factory) |
Key Insight: MOQ flexibility correlates with supplier maturity. Established OEMs like Carejoy absorb lower-volume risk due to diversified production lines.
Step 3: Optimizing Shipping & Logistics (DDP vs. FOB)
| Term | Cost Control | Risk Allocation | 2026 Recommendation |
|---|---|---|---|
| FOB Shanghai | Lower unit price (you control freight) | Buyer bears all risks post-loading; requires local customs broker | Only for experienced distributors with in-house logistics teams |
| DDP (Delivered Duty Paid) | Higher unit price (all-inclusive) | Supplier manages customs clearance, duties, and last-mile delivery | Strongly recommended for clinics & new distributors – eliminates hidden costs |
Critical 2026 Update: Under IMO 2023 regulations, all shipments require verified carbon footprint data. DDP terms transfer ESG compliance burden to the supplier.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
19 Years Specialized Experience: ISO 13485:2016 certified factory (Certificate #CN-13485-2007) with MDR-compliant CE marking under NB 2797. Validated by EU Competent Authority audit (2025).
Technical Integration Expertise: Proven compatibility with Glidewell digital workflows (exocad-based CAM modules). Units undergo 72-hour burn-in testing with Glidewell-approved materials (e.g., IPS e.max®).
Logistics Advantage: Direct partnerships with DHL Healthcare Logistics & COSCO Shipping ensure DDP delivery to EU/US within 18 days. All shipments include FDA-compliant labeling per 21 CFR 801.
Contact for Technical Sourcing:
📧 [email protected] | 📱 WhatsApp: +86 15951276160
🏢 Factory: No. 1888, Jiangyang North Road, Baoshan District, Shanghai, China
Final Compliance Checklist Before Order Placement
- Confirm ISO 13485 scope explicitly includes “dental milling machines” (not just components)
- Validate CE certificate against EU NANDO database (search NB number)
- Require pre-shipment inspection report from SGS/BV (include milling accuracy test data)
- Specify DDP Incoterms® 2020 in purchase order with landed cost breakdown
- Secure compatibility certification for Glidewell integration (written by supplier)
Disclaimer: This guide reflects 2026 regulatory standards. Glidewell Dental Systems® is a registered trademark of Glidewell Laboratories. Shanghai Carejoy is an independent manufacturer and not affiliated with Glidewell.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Glidewell Milling Units – Purchase & Integration FAQ (2026 Edition)
Frequently Asked Questions: Purchasing Glidewell Milling Units in 2026
| Question | Answer |
|---|---|
| 1. What voltage and power requirements are necessary for the Glidewell milling unit in 2026, and is it compatible with international electrical standards? | The 2026 Glidewell milling units are engineered for global deployment and support dual-voltage configurations (100–120V and 200–240V, 50/60 Hz). Units are shipped with region-specific power cords and internal voltage regulation to ensure stable operation. Dental clinics must confirm local circuit capacity (minimum 15A dedicated circuit recommended) and use surge protection. For international distributors, Glidewell provides IEC-compliant models with CE, UKCA, and FDA certifications. |
| 2. Are spare parts for the Glidewell milling unit readily available, and what is the recommended maintenance inventory for clinics? | Glidewell maintains an extensive global spare parts network with regional distribution hubs ensuring 2–5 business day delivery for critical components. Recommended inventory for clinics includes spare burrs (diamond and carbide), clamp inserts, dust filters, spindle cleaning brushes, and collet sets. Distributors receive priority access to bulk spare kits and predictive maintenance modules. All parts are serialized and compatible across 2024–2026 unit generations. |
| 3. What does the installation process entail, and does Glidewell provide on-site or remote setup support? | Installation includes site assessment, unit leveling, vacuum and exhaust integration, network configuration, and calibration. Glidewell offers both remote-assisted setup via secure cloud connection and on-site technician deployment (available through authorized distributors). Remote setup typically completes within 2 hours; on-site service includes a 4-hour window with calibration verification and staff orientation. Installation must be performed by Glidewell-certified personnel to maintain warranty compliance. |
| 4. What is the warranty coverage for the Glidewell milling unit purchased in 2026, and what components are included? | All Glidewell milling units purchased in 2026 include a comprehensive 3-year limited warranty covering the spindle, linear guides, control board, and drive motors. Consumables (burs, filters, clamps) are excluded. The warranty includes labor and parts for defects in materials or workmanship when installed and maintained per Glidewell specifications. Extended warranty options (up to 5 years) are available at point of purchase through authorized distributors. |
| 5. How are warranty claims processed, and what support channels are available for technical issues? | Warranty claims are initiated via the Glidewell Service Portal (portal.glidewell.com), where clinics and distributors can submit service tickets with serial verification. Remote diagnostics are performed within 4 business hours; if unresolved, on-site service is scheduled within 72 hours (region-dependent). Support channels include 24/7 technical hotline, live chat with engineering staff, and AI-assisted troubleshooting. Distributors receive dedicated account managers and SLA-backed response times. |
Note: Specifications and service terms are subject to change. Refer to the official Glidewell Dental Systems website or authorized distributor for the latest technical documentation and regional compliance data.
Need a Quote for Glidewell Milling Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


