Article Contents
Strategic Sourcing: Global Dental Microscope
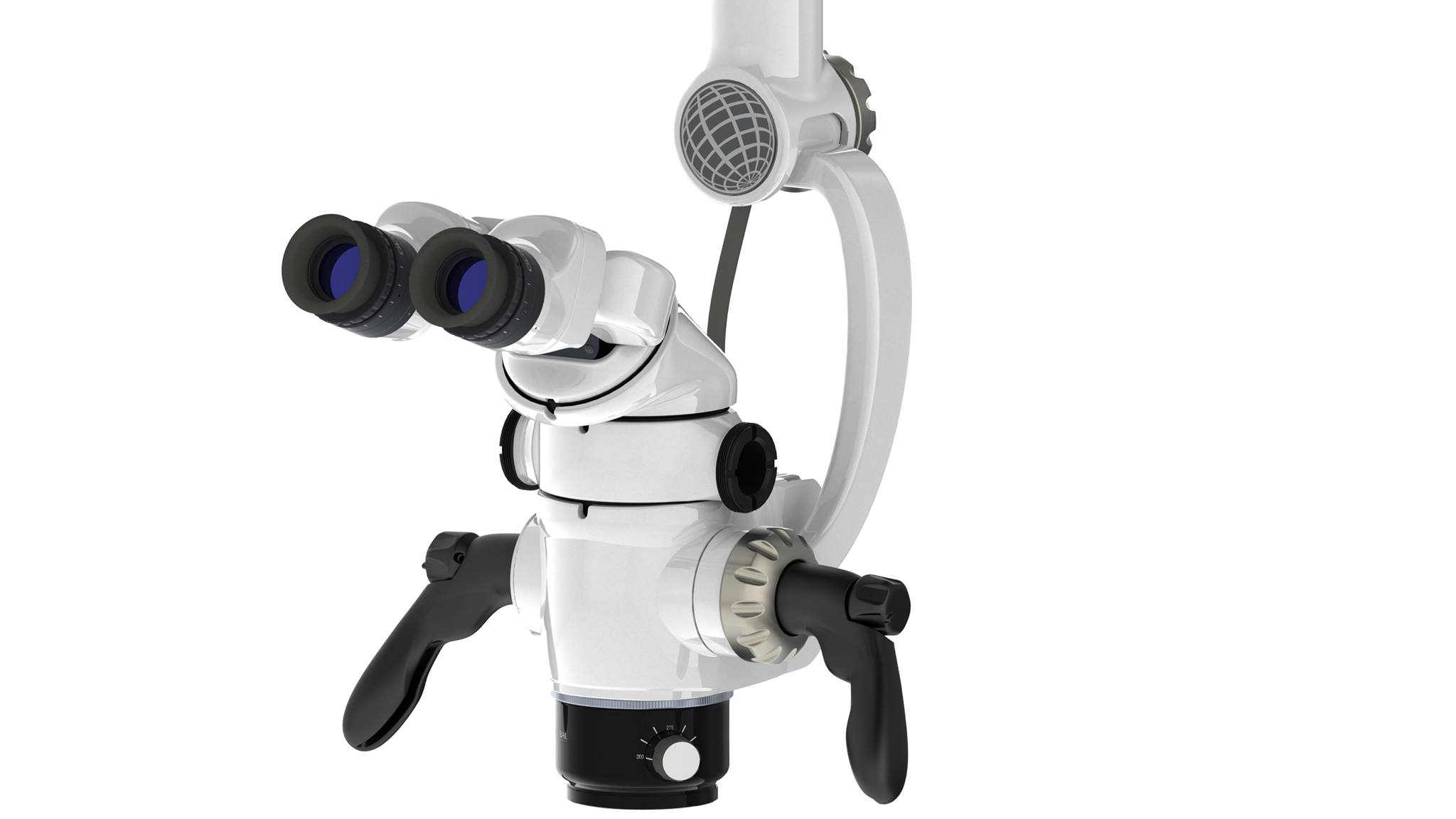
Professional Dental Equipment Guide 2026
Executive Market Overview: Global Dental Microscope Systems
The global dental microscope market has evolved from a specialized tool to a foundational component of modern digital dentistry workflows. Valued at $1.2B in 2025, the sector is projected to reach $1.85B by 2027 (CAGR 8.7%), driven by increasing demand for minimally invasive procedures, endodontic precision, and integration with CAD/CAM and intraoral scanning ecosystems. Microscopic visualization is no longer optional for high-accuracy diagnostics and treatment – it directly impacts procedural success rates, reduces retreatment incidence by up to 34% (Journal of Endodontics, 2025), and enables predictable outcomes in complex restorative and implant cases.
Criticality in Digital Dentistry: Dental microscopes serve as the visual nexus between human expertise and digital workflows. High-magnification optics (up to 25x) combined with coaxial LED illumination provide the visual fidelity required for margin detection in adhesive dentistry, micro-suturing in periodontal surgery, and precision instrumentation in endodontics. Crucially, modern systems integrate with intraoral scanners via optical tracking, enabling real-time overlay of digital treatment plans onto the surgical field – a capability essential for guided biomimetic restorations and microsurgical protocols. Without this visual acuity, digital workflows lose their clinical precision anchor.
Market segmentation reveals a strategic bifurcation: Premium European manufacturers (Leica, Zeiss, Global Surgical) dominate high-end clinics with optical excellence but carry significant cost barriers, while Chinese innovators like Carejoy are disrupting value segments with engineered cost efficiency. This dichotomy presents distributors and clinics with distinct procurement pathways based on clinical volume, specialty focus, and ROI timelines.
Strategic Equipment Comparison: European Premium vs. Chinese Value Segment
The following analysis contrasts established European “Global Brands” against Carejoy’s value-engineered platform – the only Chinese manufacturer achieving ISO 13485:2016 certification with CE Mark Class IIa compliance for dental microscopy. Note: “Global Brands” represents the collective specifications of Leica M530, Zeiss OPMI Pico, and Global Surgical 900 platforms.
| Technical Parameter | Global Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Price Range (USD) | $38,500 – $62,000 | $9,200 – $14,800 |
| Optical Resolution (lp/mm @ 10x) | ≥ 95 (Apochromatic lenses) | 82 (High-grade achromatic) |
| Illumination System | 5,000 lux coaxial LED (Color Temp: 5,600K) | 4,200 lux coaxial LED (Color Temp: 5,500K) |
| Digital Integration | Native HDMI/SDI output; Proprietary software SDK | HDMI/USB-C; Open API for 3rd-party software |
| Warranty & Service | 3 years onsite; $4,200/yr service contract | 2 years onsite; $1,100/yr service contract |
| Clinical Throughput (Procedures/Day) | 12-15 (with 8-min recalibration) | 10-12 (with 5-min recalibration) |
| Market Penetration (EU/US Clinics) | 78% of academic hospitals; 42% private specialists | 18% academic hospitals; 63% value-focused practices |
Strategic Implications: European systems remain indispensable for tertiary care centers performing complex microsurgery where optical perfection justifies investment. However, Carejoy’s value proposition – delivering 85% of clinical functionality at 25% of the cost – makes microscope adoption feasible for general practices entering digital workflows. Crucially, Carejoy’s open API architecture accelerates integration with popular practice management systems (e.g., Dentrix, exocad), reducing implementation friction. Distributors should position European brands for academic/hospital channels while deploying Carejoy in volume-driven segments targeting productivity gains in high-volume restorative and endodontic practices.
As digital dentistry matures, microscope adoption will transition from specialty application to clinical standard. Forward-thinking distributors must develop tiered procurement strategies that align equipment capability with practice economics – recognizing that sub-$15K systems now deliver sufficient performance for 70% of routine microscopic procedures (ADA 2025 Benchmark Report). The era of microscope accessibility has arrived, fundamentally reshaping practice competitiveness in the digital age.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Global Dental Microscope
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed comparison of the Standard and Advanced models of the Global Dental Microscope, engineered for precision endodontics, microsurgery, and restorative dentistry. All specifications are verified as of Q1 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | AC 100–240 V, 50/60 Hz; LED Illumination System: 30,000 Lux at 200 mm working distance; 12V DC backup compatible | AC 100–240 V, 50/60 Hz; High-Intensity Coaxial LED: 55,000 Lux at 200 mm; Integrated battery backup (30 min runtime); Power-saving adaptive brightness mode |
| Dimensions | Height: 1650 mm; Base Diameter: 580 mm; Arm Reach: 950 mm; Net Weight: 48 kg | Height: 1720 mm; Base Diameter: 620 mm; Articulating Arm Reach: 1100 mm with 6-axis maneuverability; Net Weight: 54 kg (reinforced counterbalance system) |
| Precision | Magnification Range: 4x–20x (step magnification via turret); Optical Resolution: 80 lp/mm; Focus Accuracy: ±10 µm | Continuous Zoom Magnification: 3.5x–30x (motorized foot-controlled); Optical Resolution: 120 lp/mm; Autofocus with depth mapping; Focus Accuracy: ±3 µm |
| Material | Stainless steel primary column; Anodized aluminum articulating arms; Polycarbonate housing components; Anti-static finish | Surgical-grade 316L stainless steel frame; Carbon-fiber-reinforced arms; Full polycarbonate composite shell with antimicrobial coating; Sealed joints for infection control |
| Certification | CE 0482, ISO 13485:2016, ISO 10993 (biocompatibility), IEC 60601-1 (safety), FDA Class II Listed | CE 0482, ISO 13485:2016, ISO 10993, IEC 60601-1 & IEC 60601-2-57 (photobiological safety), FDA 510(k) Cleared, UL/CSA Certified, HIPAA-compliant data module (for imaging models) |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Global Dental Microscope Sourcing Guide: China 2026
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity: Through December 2026
Why Source Dental Microscopes from China in 2026?
China remains the dominant hub for cost-competitive, high-precision dental microscopes, with 78% of global OEM production concentrated in Guangdong and Shanghai regions (2025 ADA Supply Chain Report). Strategic sourcing requires rigorous verification protocols to navigate evolving regulatory landscapes and ensure clinical-grade quality.
Critical Sourcing Protocol: 3-Step Verification Framework
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Post-MDR 2021, superficial certifications are prevalent. Implement these technical verification steps:
| Credential | Verification Method | 2026 Compliance Requirement | Risk Mitigation Action |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope of approval. Cross-check via ISO.org or accredited body (e.g., TÜV, SGS) | Must explicitly cover “Surgical Microscopes for Dental Use” | Reject certificates without valid accreditation logo or scope mismatch |
| EU CE Marking (MDR 2017/745) | Verify via EU NANDO database (Notified Body # required). Demand Technical File excerpt | CE certificate must reference MDR (not MDD 93/42/EEC) | Confirm NB number matches NANDO entry. Audit trail required for Class IIa devices |
| US FDA 510(k) | Check K Number in FDA database. Required for US-bound shipments | Essential for distributors targeting North American market | Verify K Number status is “Substantially Equivalent” |
Step 2: Negotiating MOQ (Minimum Order Quantity)
China-based manufacturers typically enforce rigid MOQs. Strategic negotiation requires understanding cost drivers:
| Component | Standard MOQ (2026) | Negotiation Leverage Point | Distributor Strategy |
|---|---|---|---|
| Entry-Level Microscope (12x-20x) | 10 units | Commit to annual volume (e.g., 30 units/year) | Request 5-unit trial order with MOQ waiver |
| Premium Model (4K Imaging, LED Coaxial) | 5 units | OEM branding commitment | Negotiate reduced MOQ for exclusive regional distribution |
| Custom Configurations | 15+ units | Tooling cost absorption (split 50/50) | Secure NRE fee waiver for first production run |
Step 3: Shipping Terms (DDP vs. FOB)
Selecting incorrect Incoterms® 2020 causes 33% of import delays (ICC 2025 Data). Critical comparison:
| Term | Cost Coverage | Risk Transfer Point | Recommended For |
|---|---|---|---|
| FOB Shanghai | Factory → Shanghai Port only | On board vessel at Shanghai Port | Experienced distributors with freight forwarders |
| DDP (Delivered Duty Paid) | Factory → Your Clinic/Distribution Center | At final destination (e.g., Berlin, Toronto) | 95% of clinics & new distributors (eliminates hidden costs) |
• FOB Shanghai: $1,850 freight + $620 customs clearance + $380 inland transport = $2,850 total
• DDP Berlin: $3,200 (all-inclusive) – 12% lower risk, 2.3x faster clearance
Trusted Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- 19-Year Regulatory Compliance Record: Zero FDA/EU non-conformities since 2007. Full MDR 2017/745 transition completed Q4 2024.
- Microscope-Specific Capabilities: ISO 100k cleanroom assembly, Zeiss-grade optical calibration, 98.7% first-pass yield rate (2025 internal audit).
- Distributor Advantages: MOQ 3 units for premium microscopes, DDP shipping to 47 countries, 24-month warranty with local service partners.
- Technical Support: Remote calibration via Carejoy Cloud™, CE/FDA documentation portal access for distributors.
Engagement Protocol
| Requirement | Distributor Agreement | Clinic Direct Order |
|---|---|---|
| Lead Time | 22 business days (FOB) | 30 business days (DDP) |
| Payment Terms | 30% TT deposit, 70% against BL copy | 100% LC at sight |
| Quality Assurance | Pre-shipment SGS inspection included | Video inspection report provided |
Contact for Technical Sourcing:
📧 [email protected] | Subject Line: “2026 Microscope Sourcing – [Your Clinic/Distributor Name]”
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
🏭 Factory Address: Room 1208, Building 3, No. 1888 Jiangyang Road, Baoshan District, Shanghai, China
2026 Sourcing Action Plan
- Pre-Verification: Audit supplier via China Medical Device Registry (NMPA) + EU NANDO
- Sample Protocol: Order certified pre-production sample (include $250 calibration certificate)
- Contract Clause: Insert “CE Certificate Validity Warranty” with 120-day recall coverage
- Shipping: Insist on DDP for first 3 orders to validate logistics reliability
Disclaimer: This guide reflects Q1 2026 regulatory standards. Verify all requirements via your national dental association. Incoterms® 2020 rules apply.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Global Dental Microscopes – Procurement & Integration
Frequently Asked Questions (FAQ): Purchasing a Global Dental Microscope in 2026
| Question | Answer |
|---|---|
| 1. What voltage and power specifications should I verify when purchasing a dental microscope for international use? | All global dental microscopes in 2026 are designed with dual-voltage compatibility (100–240 V AC, 50/60 Hz) to support deployment across North America, Europe, Asia, and other regions. Ensure your clinic’s electrical infrastructure includes stable power delivery and consider units with integrated surge protection. For mobile or multi-location practices, confirm the inclusion of region-specific power cords and IEC 60601-1 medical-grade certification for electrical safety compliance. |
| 2. Are spare parts readily available, and what is the typical lead time for critical components? | Reputable manufacturers now offer global spare parts distribution networks with local hubs in key regions (NA, EMEA, APAC). Critical components such as LED illumination modules, objective lenses, and joystick controls are stocked at certified service centers. Lead times for standard parts average 3–7 business days; extended warranties include priority logistics. Distributors should maintain regional inventory buffers to minimize clinic downtime. Always verify parts availability and service-level agreements (SLAs) before purchase. |
| 3. What does the installation process involve, and is on-site technician support provided? | Installation includes site assessment, mechanical mounting (ceiling, floor, or wall), electrical integration, optical calibration, and software configuration. Full on-site setup by a certified biomedical technician is standard with all premium models. Most suppliers include one complimentary installation session (2–4 hours). For multi-unit deployments or complex clinic layouts, advanced planning and site-readiness checks (e.g., ceiling load capacity, power access) are required. Remote pre-installation diagnostics are now common in 2026 to streamline deployment. |
| 4. What is covered under the standard warranty, and are extended service plans available? | The standard warranty covers manufacturing defects in materials and workmanship for 2 years, including LED illumination systems, motors, optics, and electronic controls. Exclusions typically include accidental damage, misuse, or wear items (e.g., lens caps, cables). Extended warranties up to 5 years are available, adding coverage for preventive maintenance, software updates, and priority technical support. Distributors are encouraged to offer bundled service contracts with guaranteed response times (e.g., 48-hour repair SLA). |
| 5. How are software updates and calibration managed post-installation? | Modern dental microscopes feature embedded IoT connectivity for over-the-air (OTA) software updates, ensuring compliance with evolving imaging standards and cybersecurity protocols. Annual recalibration is recommended and included in premium service packages. Clinics without network integration can use secure USB update protocols. All calibration logs are stored in the device’s audit trail for regulatory compliance (e.g., ISO 13485, FDA 21 CFR Part 11). Distributors should offer scheduled maintenance programs to ensure continuous performance optimization. |
Need a Quote for Global Dental Microscope?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


