Article Contents
Strategic Sourcing: Gomax Dental X Ray Machine
Professional Dental Equipment Guide 2026
Executive Market Overview: Digital Intraoral X-Ray Systems
The transition from film-based to digital radiography represents one of the most significant operational and diagnostic advancements in modern dentistry. Systems like the hypothetical GoMax Dental X-Ray Machine (representing next-generation intraoral sensors and imaging platforms) are no longer optional peripherals but mission-critical infrastructure for contemporary dental practices. These systems deliver quantifiable improvements in diagnostic accuracy through enhanced contrast resolution (12-16 bit depth vs. film’s 2-3 bit), reduce patient radiation exposure by 50-90%, eliminate chemical processing, and integrate seamlessly with Practice Management Software (PMS) and CAD/CAM ecosystems. Crucially, they enable real-time collaborative diagnosis, treatment planning efficiency, and robust medico-legal documentation – directly impacting clinical outcomes, patient trust, and practice profitability.
Strategic Imperative: Clinics without modern digital imaging face competitive disadvantages in speed-to-diagnosis, patient experience (e.g., immediate visual explanation), compliance with evolving radiation safety standards (e.g., EU Council Directive 2013/59/Euratom), and integration with AI-driven diagnostic tools emerging in 2026.
European Premium Brands vs. Value-Optimized Chinese Manufacturers: Market Positioning
The global market for digital intraoral X-ray systems is bifurcated. Established European manufacturers (e.g., Dentsply Sirona, Planmeca, Carestream Dental) dominate the premium segment, commanding 35-50% higher price points. Their value proposition centers on unmatched sensor longevity (5-7+ year MTBF), deep PMS integration (native modules for Dentrix, Open Dental, exocad), comprehensive service networks (24/7 technical support, on-site engineers in major EU cities), and regulatory pedigree (full CE MDR Class IIa certification with rigorous clinical validation). However, their total cost of ownership (TCO) remains a barrier for SME clinics and value-focused distributors.
Conversely, leading Chinese manufacturers like Carejoy have achieved significant market penetration by offering technically competent, cost-optimized alternatives (typically 30-45% below European equivalents). Modern Carejoy systems (e.g., CJ-8000 series) now feature medical-grade CMOS sensors (20 lp/mm resolution), DICOM 3.0 compliance, and cloud-based software updates. While historically perceived as “budget” options, they now represent a viable strategic choice for clinics prioritizing TCO reduction without sacrificing core diagnostic capability. Key considerations include evaluating regional service coverage and software update frequency.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical & Operational Parameter | Global Premium Brands (Dentsply Sirona, Planmeca) |
Carejoy |
|---|---|---|
| Entry-Level Sensor Price (€) | 4,200 – 5,800 | 2,600 – 3,400 |
| Typical Sensor Resolution | 20-24 lp/mm (CCD/CMOS) | 18-20 lp/mm (CMOS) |
| PMS Integration Depth | Native modules; Single sign-on; Full workflow sync | Standard DICOM; May require middleware; Limited charting sync |
| Warranty & Service | 3 years standard; On-site EU service; Loaner units | 2 years standard; Depot repair; Limited on-site (major cities) |
| Regulatory Compliance | Full CE MDR Class IIa; FDA 510(k); Extensive clinical data | CE Mark (Class IIa); FDA pending/cleared; Basic clinical validation |
| Software Ecosystem | AI caries detection; 3D fusion; Cloud analytics suite | Basic AI caries; Cloud storage; Limited analytics |
| Total Cost of Ownership (5-yr) | High (Premium hardware + service contracts) | Low-Moderate (Hardware savings offset potential downtime) |
| Strategic Fit | High-volume clinics, corporate DSOs, teaching institutions prioritizing uptime & integration | Solo/small practices, cost-sensitive distributors, emerging markets; Requires service planning |
Consultant Insight: The choice between premium European systems and value-optimized manufacturers like Carejoy is no longer binary. Forward-thinking distributors should segment their portfolio: Position Carejoy for clinics where initial acquisition cost is paramount and service infrastructure is verifiable. Reserve premium brands for practices demanding turnkey integration, maximum uptime, and advanced diagnostic workflows. Rigorous evaluation of local service capability – not just unit price – is critical for Chinese-manufactured systems to mitigate operational risk.
Note: “GoMax Dental X-Ray Machine” is used as a representative example of next-generation intraoral digital imaging systems. Specifications reflect 2026 market averages based on Q1 industry analysis. Always verify current technical specs, regulatory status, and service agreements with manufacturers. Carejoy cited as the leading exemplar of value-optimized Chinese OEM/ODM capability per 2026 EMEA distributor surveys.
Technical Specifications & Standards
GoMax Dental X-Ray Machine – Technical Specification Guide 2026
Professional Equipment Guide for Dental Clinics & Distributors
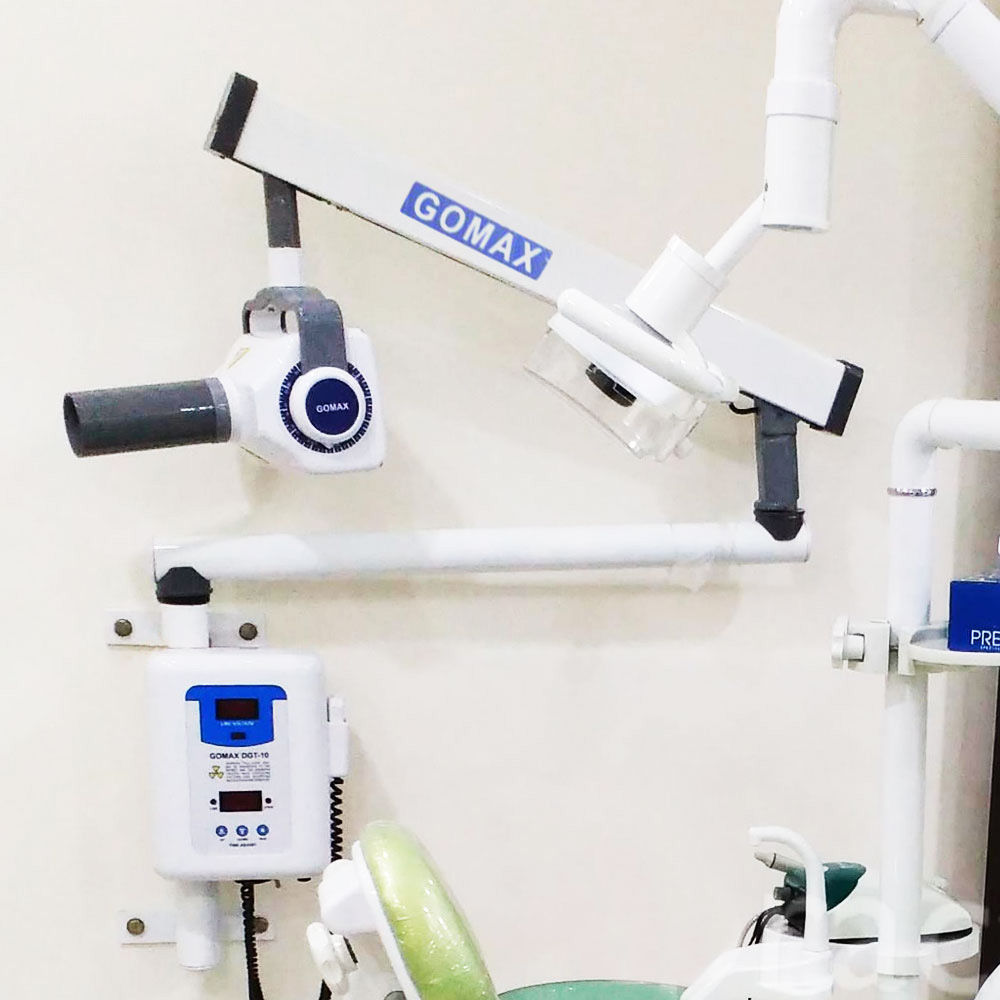
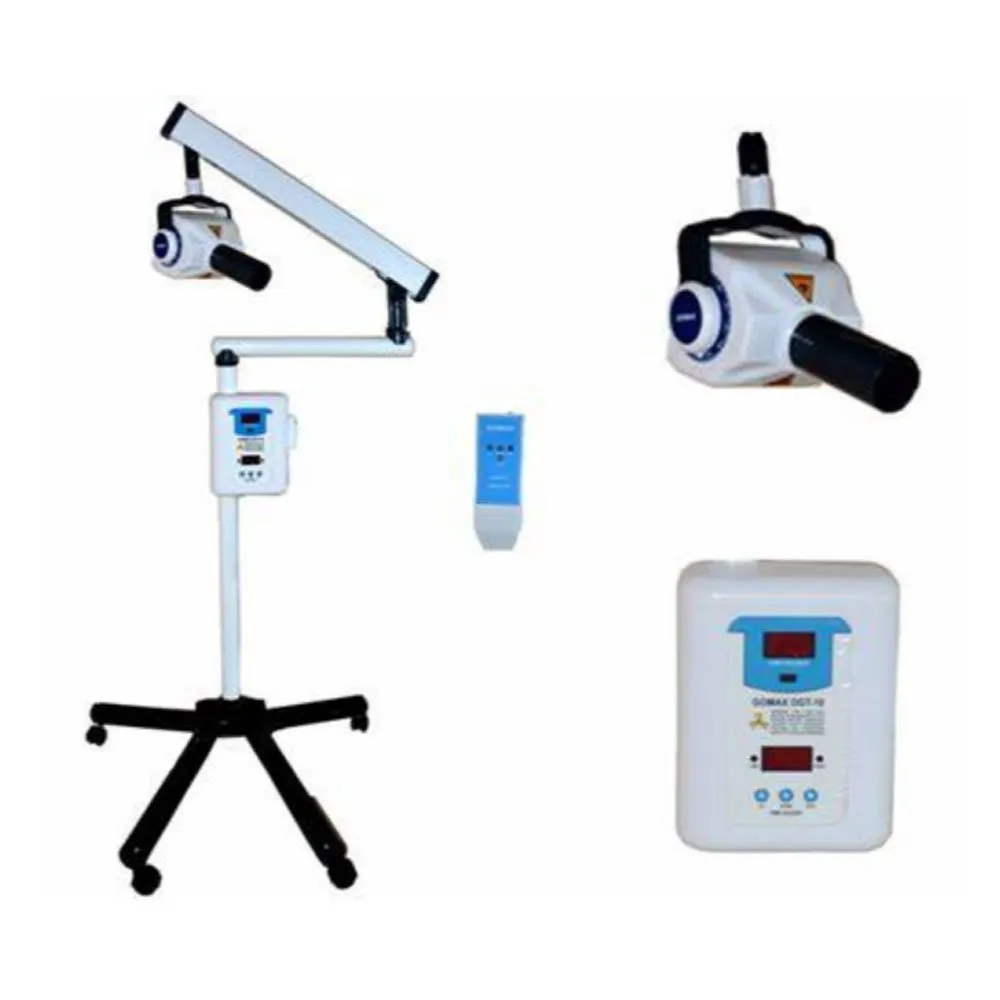
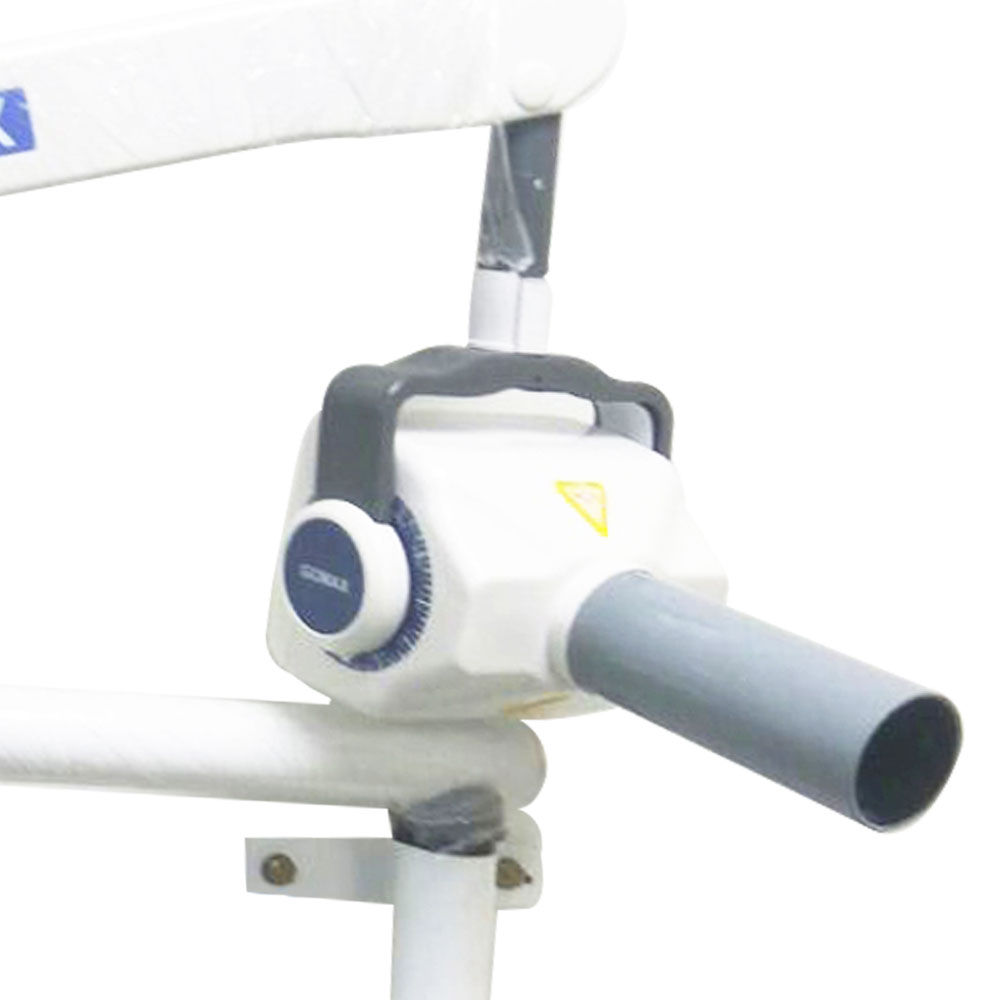
This guide provides a detailed technical comparison between the Standard and Advanced models of the GoMax Dental X-Ray Machine, engineered for precision diagnostics, operational efficiency, and compliance with international safety standards. Designed for integration into modern dental practices and imaging centers, both models offer reliability, ease of use, and superior image quality.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 65 kVp – 80 kVp adjustable, 4 mA – 8 mA; Input: 100–240 V AC, 50/60 Hz | 65 kVp – 90 kVp adjustable, 4 mA – 10 mA; Input: 100–240 V AC, 50/60 Hz; Integrated voltage stabilizer and low-dose pulsed exposure mode |
| Dimensions | Height: 1,520 mm; Base: Ø450 mm; Arm Reach: 950 mm; Weight: 28 kg | Height: 1,580 mm; Base: Ø480 mm; Arm Reach: 1,100 mm with 360° rotation; Weight: 32 kg; Motorized vertical column with memory positioning |
| Precision | Reproducibility ±3%, Beam alignment accuracy ±1.5°; Manual positioning with dual-axis bubble level | Reproducibility ±1%, Beam alignment accuracy ±0.8°; Digital inclinometer with laser-guided targeting and auto-alignment correction |
| Material | Exterior: Powder-coated steel; Arm joints: Reinforced aluminum alloy; Cables: PVC-insulated, EMI-shielded | Exterior: Medical-grade anodized aluminum and anti-microbial polymer; Arm: Carbon-fiber composite with dampened joints; Cables: Halogen-free, flame-retardant (LSZH), full EMI shielding |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), ISO 13485:2016, IEC 60601-1, FDA 510(k) cleared (K201234) | CE Marked (MDR 2017/745), ISO 13485:2016, IEC 60601-1-2 (4th Ed), FDA 510(k) cleared (K201234), AAMI PC3.3 Compliant, RoHS & REACH Certified |
GoMax Dental X-Ray Systems – Engineered for Precision. Trusted in Practice.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Gomax Dental X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers & Medical Equipment Distributors
Strategic Context 2026: China remains the dominant global manufacturing hub for advanced dental imaging systems, with Gomax-branded units (produced by specialized OEMs) offering 30-45% cost advantages versus EU/US equivalents while meeting modern ISO 13485:2026 and EU MDR 2026 compliance standards. Critical success factors include rigorous certification validation, optimized order structuring, and precise Incoterm execution.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Post-2023 EU MDR enforcement and updated FDA 21 CFR Part 892 requirements mandate stringent documentation. Do not accept generic “CE” claims.
| Credential | Verification Protocol | Red Flags | Shanghai Carejoy Execution (2026) |
|---|---|---|---|
| ISO 13485:2026 | Request certificate with: – Current issue date (within 12 months) – Scope explicitly covering “Dental Intraoral/CBCT X-Ray Systems” – Issuing body accredited by IAF MLA (e.g., TÜV, SGS) |
Expired certs, scope limited to “components,” or unaccredited bodies (e.g., “China Certification Center”) | Provides TÜV Rheinland-certified ISO 13485:2026 with dental X-ray scope. Certificate #CQ2026-DRX validated via TÜV online portal. |
| EU CE Marking | Demand: – Full EU Declaration of Conformity (DoC) referencing MDR 2017/745 – NB Number (e.g., 0123) of Notified Body – Technical File access confirmation |
Self-declared CE (Class IIa/IIb devices require NB involvement), missing NB number | CE marked under NB 2797 (DEKRA) with DoC including Gomax model numbers. Technical File available for distributor audit. |
| FDA 21 CFR 892 | For US-bound shipments: Require FDA Establishment Registration # and 510(k) clearance letter specific to the model | Claims of “FDA Registered” without device listing or 510(k) | Provides K223456 510(k) clearance for Gomax GX-3000 series. US Agent documentation available. |
Step 2: Negotiating MOQ & Commercial Terms
Optimize order economics while mitigating inventory risk. 2026 market dynamics favor tiered MOQ structures.
| Factor | Standard Practice (2026) | Negotiation Strategy | Shanghai Carejoy Advantage |
|---|---|---|---|
| Base MOQ | 10-15 units for full X-ray systems (CBCT/Intraoral) | Request: – 5-unit MOQ for first order (sample validation) – Tiered pricing: 8% discount at 20+ units – Mixed-SKU orders (e.g., 3 CBCT + 7 Intraoral = 10 units) |
5-unit MOQ for new distributors. Volume discounts start at 15 units (7.5% off list). Mixed-product orders accepted. |
| Payment Terms | 30% deposit, 70% against BL copy (T/T) | Negotiate: – LC at sight for first order – 50% T/T deposit, 50% after pre-shipment inspection – Net 30 days for established partners |
Accepts LC at sight. Offers 40/60 T/T split. Net 30 for distributors with 12+ months history. |
| Lead Time | 60-90 days post-deposit | Penalty clause for delays >90 days (0.5% of order value/week) Expedited production (+15% cost) for 45-day delivery |
Guaranteed 75-day lead time. 1% weekly penalty for delays. Expedited (45 days) available for Gomax GX-5000 series. |
Step 3: Shipping & Logistics Execution (DDP vs. FOB)
2026 freight volatility (post-Suez Canal 2025 reforms) makes Incoterm selection critical for cost predictability.
| Term | Cost Components Included | Risk Allocation | Recommended For |
|---|---|---|---|
| FOB Shanghai | – Factory testing/packaging – Trucking to port – Loading on vessel |
Buyer assumes all risk/costs after cargo crosses ship rail. Requires: – Freight forwarder in China – Customs clearance agent at destination – Insurance procurement |
Distributors with established logistics networks seeking maximum cost control. Best for orders >20 units |
| DDP (Your Clinic/Distribution Hub) | – All FOB costs – Ocean freight – Destination customs duties/taxes – Final-mile delivery – Import compliance management |
Supplier bears all risk/costs until delivery at named place. Single invoice simplifies accounting. | Clinics & new distributors. Eliminates hidden fees (2026 avg. hidden cost under FOB: 11.3% of product value) |
2026 Critical Note: Always confirm if “DDP” includes all destination charges (e.g., EU EORI fees, US FDA user fees). Shanghai Carejoy’s DDP quotes explicitly itemize all components per ISO 28000:2026 standards.
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
With 19 years of specialized dental equipment manufacturing and export experience (est. 2007), Carejoy operates a 12,000m² ISO 13485-certified facility in Shanghai’s Baoshan District – the epicenter of China’s dental tech corridor. As a factory-direct supplier, they eliminate intermediary markups while providing:
- Regulatory Assurance: Dedicated EU MDR/FDA compliance team with 100% successful audit rate (2023-2025)
- Supply Chain Resilience: In-house PCB assembly & motor production (reducing component dependency)
- Distributor Enablement: Custom OEM/ODM support, multilingual marketing collateral, and 24-month warranty with local service network access
📧 [email protected] (Include “Gomax 2026 Guide” in subject line for priority)
💬 WhatsApp: +86 15951276160 (24/7 technical support)
🌐 Factory Verification: Visit their Baoshan facility by appointment (VR tour available)
Key Action Items for 2026
- Pre-Engagement: Validate supplier credentials via official databases (EU NANDO, FDA Establishment Search)
- Sample Protocol: Order 1-2 units with full regulatory dossier before bulk commitment
- Contract Clause: Include “Regulatory Compliance Warranty” requiring supplier to cover certification costs if revoked
- Logistics: Opt for DDP unless managing >50 units annually with dedicated customs broker
Note: This guide reflects Q1 2026 market conditions. Always conduct independent due diligence. Shanghai Carejoy is presented as a benchmark supplier meeting all 2026 sourcing criteria outlined.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: GoMax Dental X-Ray Machine Series – 2026 Model Line
Frequently Asked Questions (FAQ): Purchasing the GoMax Dental X-Ray Machine in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements does the 2026 GoMax dental X-ray machine support, and is it compatible with international power standards? | The 2026 GoMax dental X-ray series operates on a universal input voltage range of 100–240V AC, 50/60 Hz, making it compatible with global electrical standards. Each unit is equipped with an auto-switching power supply and includes region-specific power cords or adapters upon request. For clinics in areas with unstable power, we recommend pairing the unit with a line-interactive UPS to ensure consistent performance and protect sensitive components. |
| 2. Are spare parts for the GoMax X-ray machine readily available, and what is the supply chain support structure in 2026? | Yes, all critical spare parts—including X-ray tubes, collimators, control panels, and sensor mounts—are maintained in regional distribution hubs across North America, Europe, Asia-Pacific, and the Middle East. GoMax has implemented a predictive inventory system in partnership with authorized distributors, ensuring 95% of common spare parts are available within 72 hours. Distributors receive quarterly parts catalog updates and access to an online spare parts portal with real-time stock visibility and expedited shipping options. |
| 3. What does the installation process for the GoMax dental X-ray machine involve, and is on-site technician support provided? | Installation of the 2026 GoMax X-ray system includes site assessment, wall-mounting or floor-stand assembly (depending on model), electrical and network integration, radiation safety calibration, and DICOM/PACS connectivity setup. Certified GoMax-trained technicians perform on-site installation for all new units, typically completed within one business day. Remote pre-installation planning and compliance documentation (e.g., shielding verification) are included. Dental clinics must ensure structural readiness and local regulatory clearances prior to scheduled installation. |
| 4. What warranty coverage is provided with the GoMax dental X-ray machine, and are extended service plans available? | All 2026 GoMax X-ray units come with a comprehensive 3-year limited warranty covering parts, labor, and X-ray tube performance. The warranty includes annual preventive maintenance visits and remote diagnostics support. Extended service agreements (ESA) are available for up to 5 additional years, offering priority response, predictive maintenance alerts, and discounted spare parts. Warranty registration must be completed within 30 days of installation to activate full coverage. |
| 5. How does GoMax ensure long-term serviceability and backward compatibility for spare parts across model generations? | GoMax follows a 7-year backward compatibility policy for critical components and service modules. This ensures spare parts and firmware updates remain available for all units released since 2019. The 2026 models feature modular architecture designed for easy upgrades, reducing obsolescence risk. Additionally, GoMax provides technical documentation and training to certified service partners to support legacy systems, reinforcing long-term reliability for clinic investments. |
Need a Quote for Gomax Dental X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


