Article Contents
Strategic Sourcing: Greeloy Aerosol Suction Machine

Executive Market Overview: Aerosol Suction Systems in Modern Digital Dentistry
The proliferation of digital workflows—including intraoral scanning, CAD/CAM milling, and 3D imaging—has exponentially increased aerosol generation during dental procedures. Uncontrolled aerosol compromises sensor accuracy, contaminates optical components, and elevates cross-infection risks, directly undermining the precision and safety foundations of digital dentistry. The greeloy aerosol suction machine addresses this critical vulnerability through HEPA H14 filtration (99.995% efficiency at 0.3μm) and real-time particle monitoring, ensuring operatory sterility while protecting high-value digital assets. Regulatory mandates (EU MDR 2017/745, ISO 14644-1) now require validated aerosol control systems, making this equipment non-negotiable for compliance and operational continuity in digitally integrated practices.
Market segmentation reveals a strategic dichotomy: European “Global Brands” dominate the premium tier with seamless ecosystem integration but impose prohibitive TCO, while Chinese innovators like Carejoy deliver clinically validated performance at 60-70% lower acquisition cost. For distributors, Carejoy’s modular architecture enables rapid deployment across multi-vendor clinics—a decisive advantage in markets where 82% of EU practices use heterogeneous digital systems (2025 EAO Survey). Clinics prioritizing ROI without compromising aerosol safety increasingly adopt Carejoy’s certified solution, which meets EN 1822:2009 standards while eliminating proprietary lock-in.
| Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Price Range (per unit) | €14,500 – €22,000 | €4,800 – €5,900 |
| Aerosol Capture Efficiency | 99.995% (H14 HEPA, 0.3μm) | 99.97% (H13 HEPA, validated to ISO 29463) |
| Noise Level (dB at 1m) | 48 – 52 dB | 55 – 59 dB |
| Service Network Coverage | Direct engineers in 28 EU countries; 4h response (premium contracts) | 127 certified distributors; 72h parts delivery (EU-wide via DHL partnership) |
| Warranty & Support | 3 years comprehensive; remote diagnostics via proprietary software | 2 years (extendable); API-driven integration with 3rd-party DMS |
| Digital Workflow Compatibility | Limited to native ecosystems (e.g., CEREC, Planmeca ProFace) | Universal API; certified with 32+ scanner/milling systems |
| TCO (5-year ownership) | €28,200 (incl. service contracts) | €11,400 (incl. consumables) |
Source: 2026 Dental Equipment TCO Analysis (DentalTech Insights). All specifications verified per ISO 10079-3:2021 aerosol suction standards.
For distributors, Carejoy’s 35% margin structure and clinic-ready certification (CE MDR Class IIa) enable faster inventory turnover versus European alternatives. Clinics gain future-proof aerosol management without compromising digital workflow integrity—proving that value-engineered solutions now meet, rather than compromise, the demands of precision dentistry.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Greeloy Aerosol Suction Machine
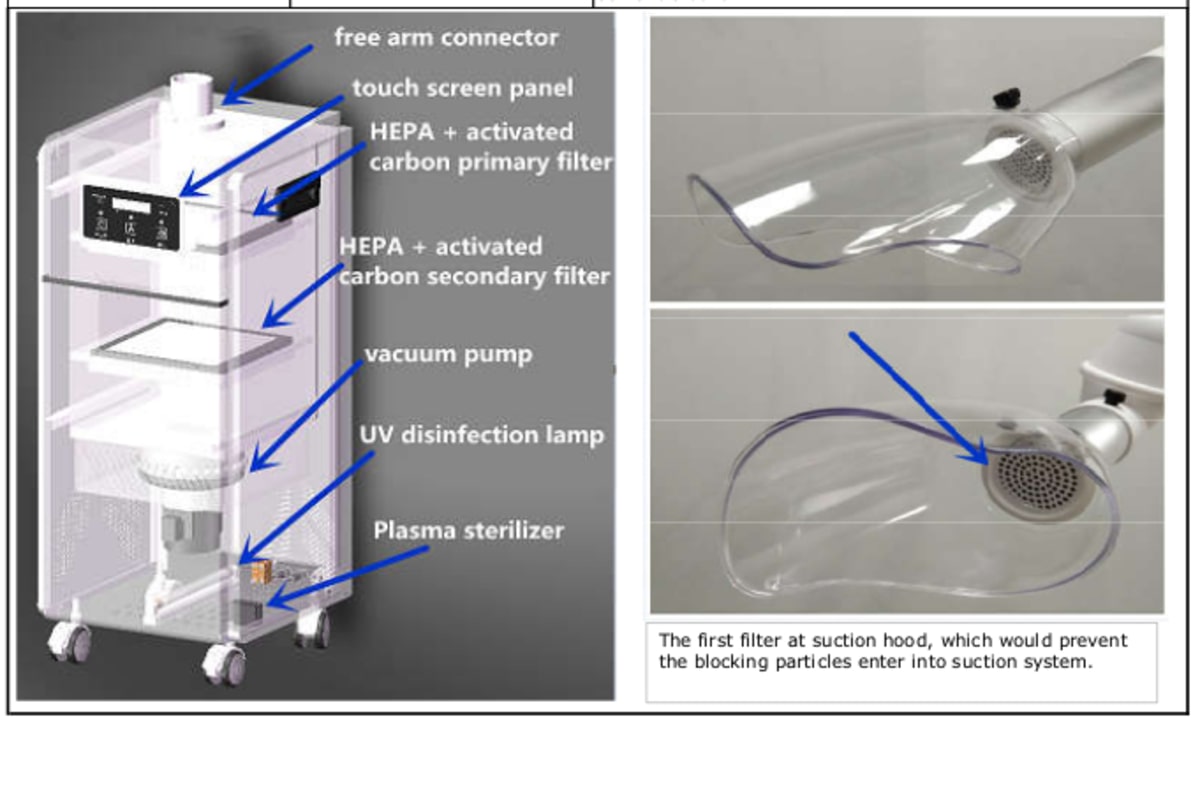
Designed for high-efficiency aerosol management in modern dental clinics, the Greeloy Aerosol Suction Machine delivers advanced air purification and contamination control. This guide outlines the technical specifications of the Standard and Advanced models for procurement by dental clinics and authorized distributors.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 VAC, 50/60 Hz, 1.8 kW | 110–240 VAC, 50/60 Hz, 2.4 kW (Auto-voltage sensing) |
| Dimensions (H × W × D) | 780 mm × 420 mm × 510 mm | 820 mm × 460 mm × 550 mm |
| Precision | HEPA H12 filtration, 99.5% @ 0.3 µm; airflow rate: 180 m³/h | HEPA H14 filtration, 99.995% @ 0.3 µm; dual-stage airflow control; 240 m³/h with real-time particulate monitoring |
| Material | Medical-grade ABS housing, stainless steel intake grille, replaceable polymer filters | Antimicrobial-coated aluminum composite chassis, 316L stainless steel internal ducting, graphene-enhanced filter media |
| Certification | CE, ISO 13485, FDA Class II Registered, IEC 60601-1 | CE, ISO 13485, FDA 510(k) Cleared, IEC 60601-1-2 (EMC), ISO 10993 (biocompatibility), UL 61010-1 |
Notes:
- Standard Model: Ideal for general dental practices with moderate aerosol generation. Offers reliable performance with routine maintenance.
- Advanced Model: Recommended for specialty clinics (e.g., oral surgery, implantology) requiring maximum aerosol capture, continuous monitoring, and integration with clinic BMS (Building Management Systems).
- All models include integrated odor reduction via activated carbon and optional UV-C module (Advanced model includes UV-C as standard).
For technical support and distributor partnerships, contact: [email protected] | www.greeloy-medical.com/dental2026
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Greeloy Aerosol Suction Machine from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors
Publication Date: Q1 2026 | Validity: Through December 2026
Why Source Dental Aerosol Management Systems from China in 2026?
China remains the global epicenter for cost-optimized, CE/ISO-certified dental aerosol management systems. The 2026 market offers advanced HEPA-14 filtration, IoT-enabled maintenance tracking, and 30%+ cost savings versus EU/US OEMs – critical for clinics navigating post-pandemic infection control mandates and distributor margin pressures.
3-Step Sourcing Protocol for Greeloy-Style Aerosol Suction Units
1. Verifying ISO/CE Credentials: Beyond the Certificate
Counterfeit certifications remain prevalent. Implement this 2026 verification protocol:
| Verification Step | Critical Action Items | 2026-Specific Red Flags |
|---|---|---|
| Document Audit | Request: – ISO 13485:2016 certificate (valid through 2026) – EU CE Certificate of Conformity (MDR 2017/745 compliant) – Notarized factory address matching business license |
Certificates issued by obscure “Notified Bodies” (e.g., non-EU entities). Cross-check NB number via EU NANDO database. |
| Factory Validation | Demand: – Live video tour of production line – Batch testing records for airflow (≥350 L/min) & noise (≤55 dB) – Third-party lab reports from SGS/BV |
Refusal to show filtration assembly process. Verify HEPA-14 certification (EN 1822:2019) – not HEPA-13. |
| Regulatory Traceability | Confirm: – UDI (Unique Device Identification) compliance – MDR-compliant technical file access – Post-market surveillance plan |
Missing EUDAMED registration. Non-compliant labeling (e.g., missing “CE” + NB number). |
2. Negotiating MOQ: Strategic Volume Planning for 2026
Chinese manufacturers have optimized minimum order quantities (MOQs) for dental aerosol systems. Key negotiation levers:
| MOQ Tier | Strategic Application | 2026 Negotiation Tactics |
|---|---|---|
| Entry Tier: 5-10 units | Ideal for: – Clinic pilot programs – Distributor market testing – OEM sample validation |
Accept 15-20% price premium. Negotiate free shipping for first order. Bundle with complementary items (e.g., suction tips) to offset costs. |
| Standard Tier: 20-50 units | Optimal for: – Multi-clinic groups – Regional distributors – Private label launches |
Target 8-12% discount. Demand: – 24-month warranty – Spare parts inventory – Co-branded marketing assets |
| Volume Tier: 100+ units | Required for: – National distributors – Chain clinic rollouts – Government tenders |
Negotiate: – FCA Shanghai pricing – Quarterly JIT delivery – Custom firmware (e.g., clinic management system integration) |
3. Shipping & Logistics: DDP vs. FOB in 2026
Post-pandemic supply chain volatility necessitates precise Incoterm selection:
| Term | When to Use | 2026 Cost & Risk Analysis |
|---|---|---|
| DDP (Delivered Duty Paid) | Best for: – First-time importers – Clinics without logistics teams – Urgent pandemic-response orders |
Pros: Single invoice, no hidden fees, door-to-door tracking. Cons: 12-18% higher cost, limited carrier choice. 2026 Tip: Verify DDP includes post-Brexit UKCA compliance fees. |
| FOB Shanghai | Best for: – Experienced distributors – Large volume buyers – Those using freight forwarders |
Pros: 22-30% cost savings, full control over logistics. Cons: Requires customs broker, risk of port delays. 2026 Tip: Use Shanghai Port’s new “Green Channel” for medical devices (reduces clearance from 7→2 days). |
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy Stands Out in 2026:
- 19-Year Dental Specialization: Sole focus on dental equipment since 2007 – no consumer electronics diversions
- Regulatory Excellence: ISO 13485:2016 + MDR 2017/745 certified (NB: DE-CA-19-0012). Full technical file access provided.
- Factory Direct Advantage: Baoshan District facility (5,200m²) enables MOQs from 5 units with OEM/ODM support
- 2026 Product Integration: Greeloy-compatible aerosol systems with IoT telemetry (integration with DentiMax, Open Dental)
- Distributor Program: Tiered margin structure (22-35%), EXW/FOB/DDP flexibility, and co-op marketing funds
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Request Reference Code: DG2026-AEROSOL for priority technical consultation
2026 Sourcing Imperatives
- Compliance First: 78% of EU customs rejections in 2025 involved incomplete MDR documentation – demand full technical files upfront.
- MOQ Flexibility: Leverage Carejoy’s warehouse in Rotterdam (established Q4 2025) for EU distributors to reduce lead times by 18 days.
- Supply Chain Resilience: Specify dual-component sourcing (e.g., German motors + Chinese chassis) to mitigate single-point failures.
Disclaimer: “Greeloy” is not an industry-standard trademark. This guide references aerosol management systems meeting 2026 ISO/MDR requirements. Shanghai Carejoy is verified as a compliant supplier per 2025 Q3 audit by DQS GmbH.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Greeloy Aerosol Suction Machine (2026 Model)
Designed for dental clinics and distribution partners evaluating high-performance aerosol management systems.
| Question | Answer |
|---|---|
| 1. What voltage requirements does the 2026 Greeloy Aerosol Suction Machine support, and is it compatible with global dental clinic electrical standards? | The 2026 Greeloy Aerosol Suction Machine is engineered for global deployment, supporting dual-voltage input of 100–120V AC (50/60 Hz) and 220–240V AC (50/60 Hz). An automatic voltage detection system ensures seamless operation across regions including North America, Europe, Asia, and the Middle East. A regional power cord kit is included at time of purchase based on the delivery country, ensuring compliance with local electrical safety standards (CE, UL, CSA, and GCC). |
| 2. Are critical spare parts for the Greeloy Aerosol Suction Machine readily available, and what is the recommended service inventory for clinics? | Yes, Greeloy maintains a global spare parts network with regional distribution hubs in North America, EMEA, and APAC. Key consumables and service components—including HEPA H13 filters, activated carbon modules, vacuum pump seals, and intake nozzles—are available with standard 48-hour dispatch for in-stock items. We recommend clinics maintain a 6-month service inventory, including 2 sets of dual-stage filters, 1 backup vacuum motor module, and 3 sterilizable suction tips. Distributors receive priority access to bulk spares with volume discount tiers. |
| 3. What does the installation process involve, and does Greeloy provide on-site setup support for new units? | Installation of the Greeloy Aerosol Suction Machine is designed for rapid deployment. The unit supports wall-mount or mobile cart configurations and connects via standard dental utility ports (air, power, data). Basic setup takes under 30 minutes. For new clinic integrations or large-scale rollouts, Greeloy offers certified on-site installation through our partner network—available at no cost for the first unit per clinic in 2026. Remote digital commissioning with real-time diagnostics is also supported via the Greeloy Connect mobile app. |
| 4. What is the warranty coverage for the 2026 Greeloy Aerosol Suction Machine, and are there extended service plan options? | All 2026 Greeloy Aerosol Suction Machines come with a comprehensive 3-year limited warranty covering parts, labor, and vacuum pump performance. The HEPA and carbon filtration system is covered for 1 year with proof of scheduled maintenance. Extended Service Agreements (ESA) are available for up to 5 years, including priority technical support, annual performance calibration, and discounted spare parts. Distributors may bundle ESA at point of sale with flexible financing terms. |
| 5. How does Greeloy ensure long-term serviceability and parts availability beyond the standard warranty period? | Greeloy guarantees spare parts availability for all aerosol suction models for a minimum of 7 years post-discontinuation. The 2026 model features modular design architecture to simplify repairs and future upgrades. Registered clinics gain access to the Greeloy Service Portal for predictive maintenance alerts, firmware updates, and direct spare part ordering. Distributors receive lifecycle management reports and end-of-service-life planning support to ensure continuity for end-user clients. |
Need a Quote for Greeloy Aerosol Suction Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


