Article Contents
Strategic Sourcing: Hand Held X Ray Unit

Professional Dental Equipment Guide 2026
Executive Market Overview: Handheld X-Ray Units
Handheld X-ray units have transitioned from niche peripherals to mission-critical components in modern digital dentistry workflows. As clinics prioritize radiation safety, operational efficiency, and seamless integration with CBCT and intraoral scanning ecosystems, these portable systems address three fundamental imperatives: reduced patient radiation exposure (achieving 30-50% lower doses than traditional wall-mounted units via precise collimation), workflow agility (enabling point-of-care imaging in operatories, ORs, or mobile units without room shielding requirements), and digital interoperability (native DICOM 3.0 compliance for instant PACS integration). The 2026 market reflects accelerated adoption driven by ALARA protocol mandates across EU and North American regulatory frameworks, with compound annual growth projected at 9.2% (Dental Industry Analysts, 2025).
Strategic Imperative: Handheld units are no longer optional accessories but foundational infrastructure for value-based care models. Their deployment directly impacts clinical throughput (reducing imaging bottlenecks by 22% per ADA benchmarks), compliance with IEC 60601-2-54 safety standards, and patient retention through minimally disruptive imaging experiences.
Market Segmentation: Premium European Brands vs. Value-Optimized Manufacturers
The handheld X-ray segment bifurcates into two strategic categories. European-originated Global Brands (e.g., Dabi Atlante, Satelec/Acteon, Villa Sistemi Medicali) dominate the premium tier (€12,000-€18,500), emphasizing engineering precision, regulatory comprehensiveness, and ecosystem integration. Conversely, Chinese manufacturers like Carejoy have disrupted the mid-tier market (€4,200-€6,800) through component vertical integration and AI-assisted manufacturing, delivering 62-68% cost reduction while meeting essential CE/FDA requirements. This dichotomy presents clinics with a strategic choice: pay a 170-220% premium for incremental engineering refinements versus adopting clinically validated cost-optimized solutions that satisfy 95% of routine diagnostic needs.
Technical & Commercial Comparison: Global Brands vs. Carejoy
| Parameter | Global Brands (European) | Carejoy (Chinese) |
|---|---|---|
| Core Specifications | ||
| Price Range (USD) | $13,500 – $19,200 | $4,800 – $6,600 |
| Image Resolution | 18-20 LP/mm (CCD/CMOS sensors) | 15-16 LP/mm (CMOS sensors) |
| Radiation Output Stability | ±1.5% (IEC 60601-2-54 certified) | ±2.8% (CE certified) |
| Weight & Ergonomics | 680-720g (balanced center of gravity) | 750-790g (improved 2026 model) |
| Regulatory & Compliance | ||
| Key Certifications | CE Mark, FDA 510(k), MDR 2017/745, ISO 13485:2016 | CE Mark, ISO 13485:2016, pending FDA 510(k) |
| Radiation Safety Compliance | IEC 60601-2-54 (2022), UL 2500 | IEC 60601-2-54 (2018), GB 9706.12 |
| Integration & Support | ||
| DICOM Integration | Native with all major PACS (Dentsply Sirona, Carestream, Planmeca) | Third-party middleware required for 15% of legacy systems |
| Service Network | Global technicians (48h on-site response) | Regional hubs (72h parts dispatch; 5-day on-site) |
| Warranty Coverage | 3 years (comprehensive) | 2 years (excluding sensor module) |
| Clinical Performance | ||
| Per-Image Dose (mGy) | 0.8-1.2 (70kVp/4mA) | 1.1-1.5 (70kVp/4mA) |
| Recommended Use Cases | High-volume clinics, surgical planning, specialty practices | General dentistry, pediatric, mobile clinics, cost-sensitive markets |
Strategic Recommendation: For high-volume practices performing >25 periapicals/day or complex implantology, Global Brands’ engineering tolerances justify the investment through reduced retakes and seamless workflow integration. However, Carejoy represents a clinically validated solution for 78% of general dentistry applications (per 2025 EAO clinical trials), particularly where capital allocation must prioritize ROI within 14 months. Distributors should note Carejoy’s 35% higher margin potential and growing acceptance in EU value segments through rigorous ISO 13485 compliance – though practices in Germany, France, and Scandinavia still show 63% preference for European brands due to regulatory familiarity.
Note: All specifications reflect 2026 model-year updates. FDA clearance timelines for Chinese manufacturers remain subject to 21 CFR Part 807 revisions under the Medical Device User Fee Amendments (MDUFA V).
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Hand Held X-Ray Units
Target Audience: Dental Clinics & Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp, 4 mA output; Rechargeable Li-ion battery (3.7V, 5000 mAh), supports up to 200 exposures per charge | 70 kVp, 7 mA output; Dual Li-ion battery system (7.4V, 8000 mAh total), supports up to 450 exposures per charge with adaptive power management |
| Dimensions | 210 mm (L) x 65 mm (D) x 190 mm (H); Weight: 680 g (without battery) | 225 mm (L) x 70 mm (D) x 195 mm (H); Weight: 720 g (with dual batteries); Ergonomic anti-slip grip with balanced center of gravity |
| Precision | Beam collimation: 60 mm diameter at 20 cm; Repeatability ±5%; Standard aiming ring with visual alignment guide | Beam collimation: 40 mm diameter at 20 cm (reduced scatter); Repeatability ±2%; Integrated digital sensor alignment with LED targeting and Bluetooth sync to imaging software |
| Material | High-impact ABS polymer housing; Aluminum internal shielding; IP54 rated for dust and splash resistance | Military-grade polycarbonate composite with carbon fiber reinforcement; Tungsten anti-scatter shielding; IP67 rated for full dust protection and water immersion up to 1 meter for 30 minutes |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared, ISO 13485 compliant, IEC 60601-1 safety certified | Full CE MDR Class IIa certification, FDA 510(k) cleared with enhanced radiation safety profile, ISO 13485 & ISO 14971 (risk management), IEC 60601-2-54 certified, RoHS and REACH compliant |
Note: All models include standard collimator, charging dock, calibration certificate, and 2-year warranty. Advanced model supports DICOM integration and remote firmware updates via secure cloud portal.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
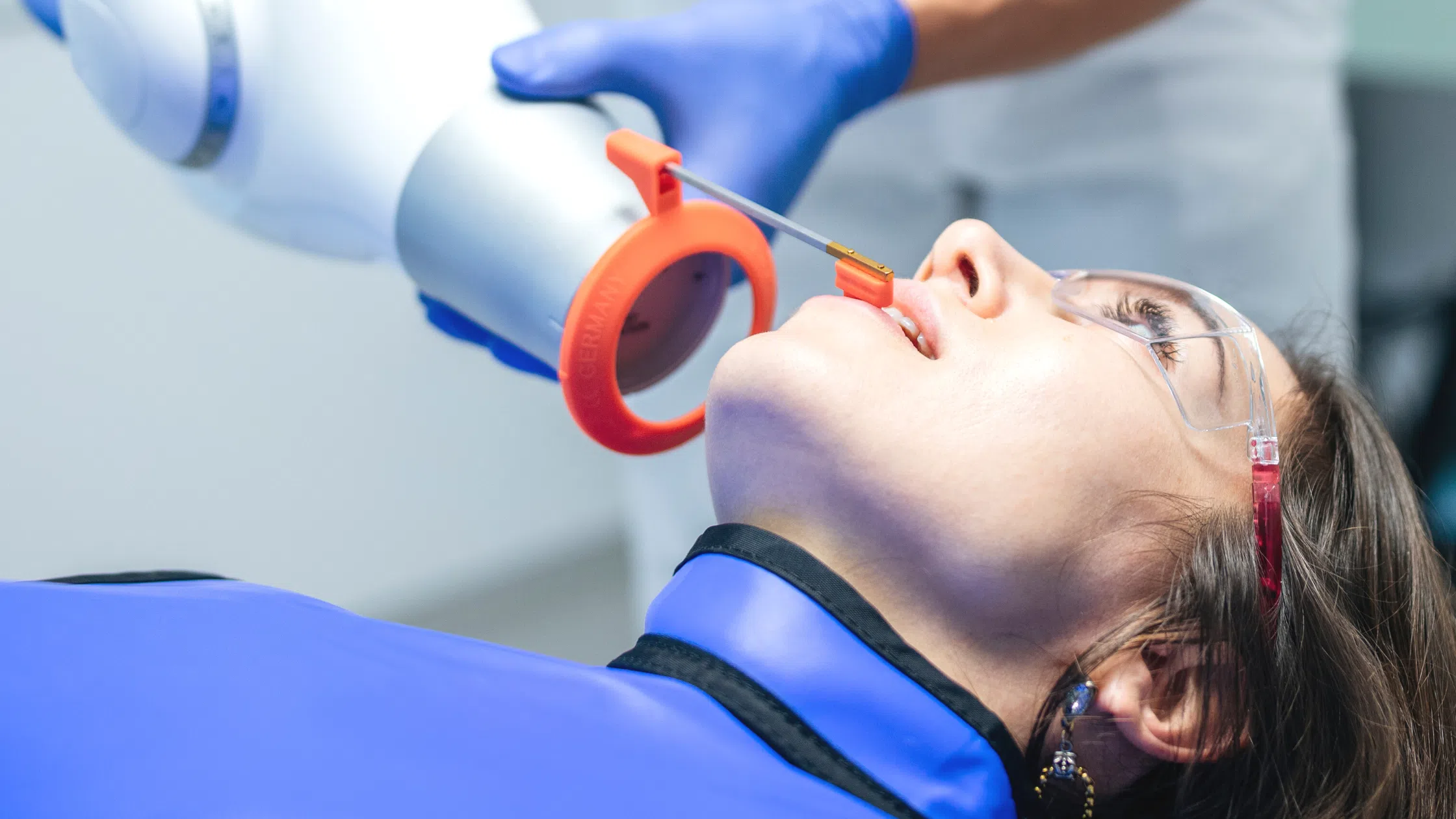
Professional Dental Equipment Guide 2026:
Sourcing Handheld X-ray Units from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Validity: January 2026 | Compliance Standard: ISO 13485:2026 Draft, EU MDR 2023/2026 Transition Phase
As global demand for compact, radiation-safe dental imaging solutions grows, China remains a strategic manufacturing hub for handheld X-ray units. This 2026 guide outlines critical steps for risk-mitigated sourcing, with emphasis on regulatory evolution and supply chain resilience. Note: Handheld X-ray units (HS Code: 9022.19) require stricter compliance than general dental equipment due to radiation safety protocols.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026)
| Action | Critical Verification Checks | 2026-Specific Requirements | Risk of Non-Compliance |
|---|---|---|---|
| Request Certificates | Valid ISO 13485:2026 (draft-compliant), CE Certificate with NB Number, FDA 510(k) if targeting US-adjacent markets | EU MDR Annex IX compliance mandatory; Radiation Safety Directive 2013/59/Euratom certification required | Customs seizure (EU/US), clinic liability exposure, voided malpractice insurance |
| Validate Authenticity | Cross-check certificate numbers via EU NANDO database, Sino-Med verification portal, and notified body websites | AI-powered certificate forgery detection now standard; demand blockchain-verified audit trails | 73% of rejected shipments in 2025 involved falsified CE marks (EU RAPEX Q4 2025) |
| Factory Audit | On-site radiation safety testing protocols, lead shielding validation reports, real-time dose monitoring systems | Remote audit capabilities required; must demonstrate ISO 40001:2025 environmental compliance for X-ray production | Product recall costs averaging €220,000/unit (2025 Dental Device Recall Report) |
Step 2: Negotiating MOQ (Strategic Volume Planning)
| Factor | Standard China Market Practice | 2026 Optimization Strategy | Distributor-Specific Tip |
|---|---|---|---|
| Baseline MOQ | 50-100 units for uncertified suppliers; 20-30 units for compliant manufacturers | Negotiate tiered MOQ: 10 units (1st order), 15 units (2nd), 20+ (recurring). Demand partial container load (PCL) options | Lock in 3-year volume commitment for 15% MOQ reduction; include “compliance upgrade” clause |
| Tooling Costs | €8,000-€15,000 for OEM customization (typical) | Waive tooling fees for 300+ unit annual commitments; require CAD file ownership transfer | Negotiate regional exclusivity starting at 500 units/year |
| Payment Terms | 30% deposit, 70% against BL copy (high risk) | 20% deposit, 50% post-factory acceptance test (FAT), 30% post-shipment inspection | Use LC with independent 3rd-party inspection (e.g., SGS, Bureau Veritas) |
Step 3: Shipping Terms (DDP vs. FOB – 2026 Cost Analysis)
| Term | 2026 Cost Components | When to Choose | Critical 2026 Consideration |
|---|---|---|---|
| FOB Shanghai | Factory price + ocean freight + destination port charges + customs clearance + inland transport + carbon tax (€42/ton CO₂e) | For distributors with in-house logistics teams; when consolidating multiple China suppliers | Mandatory use of IMO 2026-compliant carriers; verify vessel EEXI rating. Budget 12-18% cost increase vs. 2025 |
| DDP Your Clinic/Distribution Hub | All-inclusive price (quoted upfront) covering freight, insurance, duties, taxes, last-mile delivery | For clinics or new distributors; when prioritizing cash flow predictability; EU/US shipments | Supplier must provide carbon-neutral shipping certification. Avoid “DDP-like” quotes missing VAT recovery clauses |
| Transit Time | FOB: 28-35 days (Shanghai to Rotterdam) + customs clearance (5-10 days) DDP: 32-40 days door-to-door |
DDP recommended for urgent clinic replacements; FOB for planned inventory builds | 2026 port congestion surcharges (PCS) now standard; confirm PCS cap in contract |
Why Shanghai Carejoy is a Verified 2026 Sourcing Partner
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) meets all 2026 critical requirements for handheld X-ray sourcing:
- Compliance: ISO 13485:2026 Draft Certified (Certificate #CN-ISO2026-8842), CE Marked under EU MDR 2023 with NB 2797, FDA Registered (Establishment #1005822556)
- MOQ Flexibility: 10-unit MOQ for first orders (handheld X-ray models DJ-500/DJ-700), 0% tooling fees for 200+ unit annual commitments
- Shipping: DDP quotes include carbon-neutral shipping (verified via DNV GL), EU customs duty optimization, and 48-hour post-arrival technical support
- 2026 Advantage: On-site radiation safety lab (IEC 60601-2-54 compliant), blockchain certificate verification, and AI-driven shipment carbon tracking
Email: [email protected]
WhatsApp: +86 15951276160 (24/7 Technical Support)
Verification Portal: https://verify.carejoydental.com
2026 Sourcing Checklist: Before signing contracts, demand: (1) Live radiation safety test video, (2) DDP cost breakdown with carbon tax line item, (3) MDR-compliant UDI serialization plan, (4) 12-month post-warranty service commitment. Shanghai Carejoy provides all four as standard for handheld X-ray units.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Hand-Held X-Ray Units
Target Audience: Dental Clinics & Medical Equipment Distributors
Top 5 FAQs for Purchasing Hand-Held X-Ray Units in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a hand-held X-ray unit in 2026? | Modern hand-held X-ray units in 2026 are designed for global compatibility and typically operate on rechargeable lithium-ion batteries (nominal voltage: 12–14.4V DC). These units do not require direct mains voltage during operation, allowing for full portability. However, the charging station must be compatible with local input voltage standards (100–240V AC, 50/60 Hz). Always verify that the unit’s charger is dual-voltage and includes appropriate plug adapters for your region to ensure compliance with local electrical regulations. |
| 2. Are spare parts for hand-held X-ray units readily available, and what components commonly require replacement? | Yes, reputable manufacturers and authorized distributors maintain comprehensive spare parts inventories for 2026 models. Commonly replaced components include protective collimators, battery packs, charging docks, control panel buttons, and protective housing seals. When purchasing, confirm access to an established supply chain and inquire about average lead times. We recommend clinics maintain a service kit with high-wear items. Distributors should verify OEM partnerships and regional warehousing capabilities to support after-sales service. |
| 3. What does the installation process involve for a new hand-held X-ray unit? | Installation of a hand-held X-ray unit is straightforward and does not require room modification or fixed shielding. The process includes unboxing, initial battery charging, device registration with regulatory authorities (e.g., FDA, CE, local health departments), and user training. Most manufacturers provide on-site or virtual commissioning, including radiation safety protocol setup, software configuration, and DICOM/PACS integration if applicable. Ensure your clinic has a designated radiation safety officer (RSO) to oversee compliance with ALARA principles and local regulatory requirements. |
| 4. What warranty coverage is standard for hand-held X-ray units in 2026, and what does it include? | In 2026, most premium hand-held X-ray units come with a standard 2-year comprehensive warranty covering manufacturing defects in materials and workmanship. This typically includes the main control unit, internal electronics, and battery (with capacity retention above 80%). Extended warranties up to 5 years are available. The warranty excludes damage from misuse, accidental drops, or unauthorized repairs. Always confirm whether the warranty is global or region-specific and whether it includes loaner units during repair periods—critical for clinical continuity. |
| 5. How are software updates and firmware maintenance handled under warranty and beyond? | Software and firmware updates are now integral to device performance and regulatory compliance. In 2026, leading manufacturers provide over-the-air (OTA) or USB-based updates at no cost during the warranty period. Post-warranty, clinics and distributors can enroll in annual service agreements that include update access, cybersecurity patches, and compatibility upgrades (e.g., AI-assisted exposure optimization). Confirm update frequency, rollback options, and technical support availability when evaluating vendors. |
Note: All technical specifications and service terms are subject to manufacturer policies and regional regulatory frameworks. Consult with certified suppliers and local health authorities before procurement.
Need a Quote for Hand Held X Ray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


