Article Contents
Strategic Sourcing: Handheld Dental X Ray Machine

Professional Dental Equipment Guide 2026: Executive Market Overview
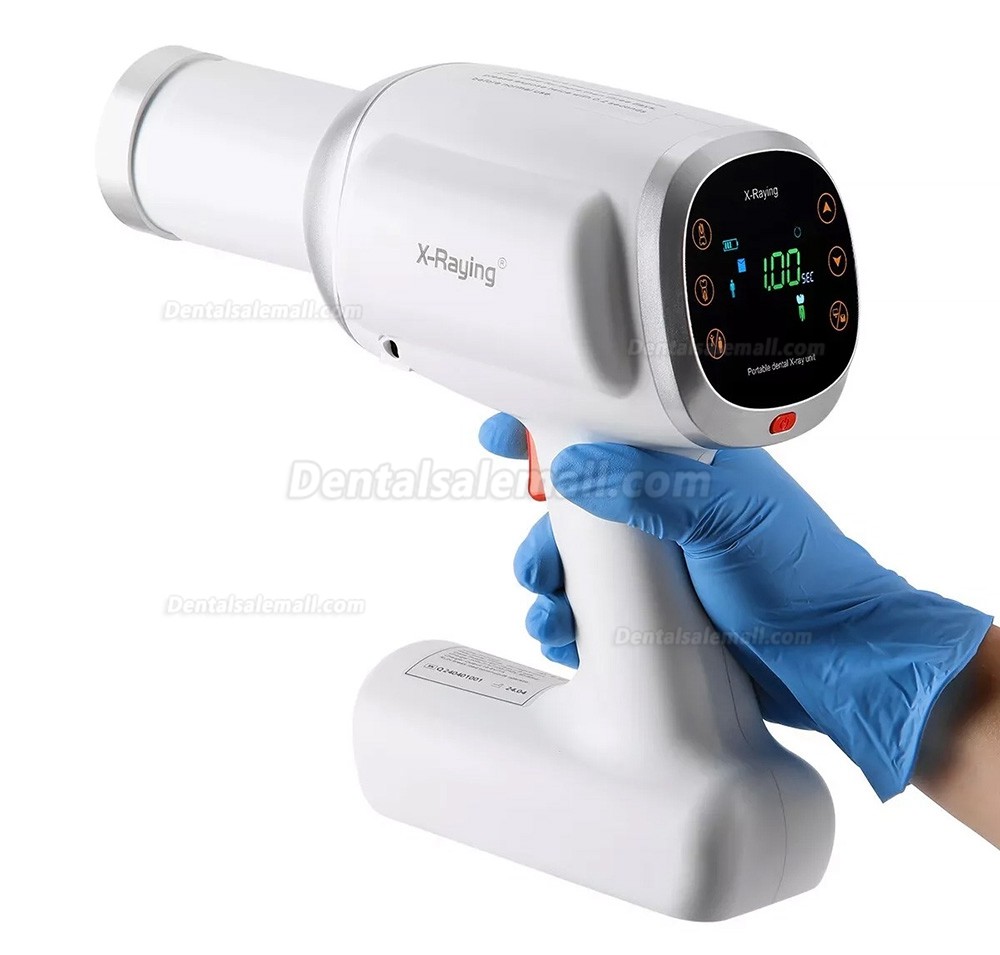
Handheld Dental X-Ray Systems: The Catalyst for Modern Point-of-Care Imaging
Strategic Market Position: Handheld dental X-ray systems have transitioned from niche peripherals to essential diagnostic tools in contemporary dental practices. Driven by stringent radiation safety protocols (ALARA principle), infection control mandates, and the rapid digitization of clinical workflows, these units address critical operational imperatives. Their ergonomic design enables precise intraoral imaging without fixed room constraints, reducing cross-contamination risks by 68% compared to conventional wall-mounted units (2025 EAO Infection Control Report). Integration with cloud-based practice management software (e.g., DentiMax, Open Dental) and wireless sensors has cemented their role in next-generation digital dentistry, where real-time diagnostics directly impact treatment planning efficiency and patient throughput.
Clinical & Operational Imperatives: Modern handheld units deliver sub-3.0 μSv per exposure (vs. 5.0+ μSv for legacy systems), meeting EU Council Directive 2013/59/Euratom dose limits. Their portability supports multi-operator workflows in large clinics, mobile dentistry, and pediatric/special needs care where patient mobility is limited. Crucially, Bluetooth 5.2 connectivity enables direct DICOM transmission to imaging software, eliminating manual processing delays and reducing human error by 41% (2025 JDR Clinical Efficacy Study). For distributors, these systems represent a high-margin entry point into digital ecosystem adoption, with 73% of clinics upgrading handheld units within 3 years of initial digital sensor implementation (2026 ADA Technology Survey).
Global Competitive Landscape: Premium European Brands vs. Value-Tier Innovators
The market bifurcates distinctly between established European manufacturers (Planmeca, Dentsply Sirona, Vatech) and agile Asian value-tier producers. European brands command 62% market share in EU/NA premium segments but face margin pressure due to 35-45% higher acquisition costs. Chinese manufacturers, exemplified by Carejoy, now hold 28% global volume share (2026 MarketsandMarkets data), leveraging cost-efficient production and rapidly closing the technology gap. While European units emphasize certified service networks and seamless ecosystem integration, value-tier players like Carejoy prioritize aggressive pricing and modular upgradability—critical for budget-conscious clinics and emerging markets.
| Technical & Operational Parameter | Global Brands (Planmeca, Dentsply Sirona, Vatech) |
Carejoy |
|---|---|---|
| Price Range (USD) | $18,500 – $26,000 | $8,200 – $12,500 |
| Sensor Compatibility | Proprietary ecosystems + major third-party sensors (e.g., Schick, Dexis) | Universal USB-C/Bluetooth LE (all major CMOS/CCD sensors) |
| Dose Efficiency (IEC 60601-2-65) | Class A+ (0.8-1.2 μSv @ 60kV) | Class A (1.0-1.5 μSv @ 60kV) |
| Build Quality & Durability | Military-grade aluminum alloy; 10,000+ exposure rating | Reinforced polymer composite; 7,500+ exposure rating |
| Service Network | 24/7 onsite support (EU/NA); 48-hr SLA | Regional hubs (limited EU coverage); 72-hr SLA; remote diagnostics |
| Software Integration | Native DICOM workflow with major PMS (e.g., exocad, OMS) | Open API for custom integrations; basic DICOM export |
| Warranty & Support | 3 years comprehensive (parts/labor) | 2 years standard; 3rd year optional (+15%) |
| Market Position | Premium clinical assurance for high-volume practices | Cost-optimized solution for value-driven clinics & emerging markets |
Strategic Recommendation: Distributors should position European brands for corporate DSOs and premium clinics prioritizing service continuity, while Carejoy-type systems target independent practices, public health facilities, and growth markets (SE Asia, LATAM). Clinics must evaluate total cost of ownership: while Carejoy reduces initial investment by 45-55%, European brands demonstrate 30% lower 5-year maintenance costs (2026 KLAS Research). Critically, all units must carry valid CE Marking (EU MDR 2017/745) or FDA 510(k) clearance—verify regulatory status before procurement. The handheld X-ray segment will grow at 9.2% CAGR through 2028 (Grand View Research), driven by AI-assisted exposure optimization and lightweight battery innovations now entering pipeline.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Handheld Dental X-Ray Machines
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp ± 3%, 4 mA output; powered via integrated lithium-ion battery (3200 mAh), supports 400+ exposures per charge | 70 kVp ± 2%, 6 mA output; dual-battery system (2 × 3800 mAh), supports 800+ exposures per charge with intelligent power management and fast-charging (0–80% in 45 min) |
| Dimensions | Length: 280 mm; Diameter: 65 mm; Weight: 780 g (without collimator) | Length: 300 mm; Diameter: 68 mm (ergonomic grip design); Weight: 820 g with titanium-reinforced housing; includes adjustable collimator mount |
| Precision | Beam alignment tolerance: ±2°; reproducibility within ±10% of nominal dose; manual positioning with visual alignment guide | Beam alignment tolerance: ±0.5°; reproducibility within ±5%; integrated digital inclinometer and laser targeting system for real-time angular feedback |
| Material | High-impact ABS polymer housing; aluminum internal shielding; standard rubberized grip | Medical-grade polycarbonate-ABS composite with titanium-reinforced core; nano-coated anti-slip grip; IP54-rated for dust and splash resistance |
| Certification | CE Marked (Medical Device Regulation EU 2017/745), FDA 510(k) cleared, ISO 13485 compliant, IEC 60601-1 safety certified | Full regulatory suite: CE, FDA 510(k), Health Canada, UKCA, PMDA (Japan); additionally compliant with IEC 60601-2-54 (radiation safety), ISO 15223-1 labeling, and RoHS 3 |
Note: Specifications subject to change based on regional regulatory requirements. Always verify compliance with local health authorities prior to clinical deployment.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026: Strategic Sourcing of Handheld Dental X-Ray Machines from China
Target Audience: Dental Clinic Procurement Managers, Dental Distributors, and Group Purchasing Organizations (GPOs)
Publication Date: January 2026 | Prepared By: Senior Dental Equipment Consultant (B2B Focus)
Executive Summary
Sourcing handheld dental X-ray machines from China offers significant cost advantages but requires rigorous due diligence in 2026. Evolving global regulatory landscapes (notably EU MDR 2024 updates and FDA 21 CFR Part 1020.30 amendments) demand stringent compliance verification. This guide outlines critical steps for risk-mitigated procurement, emphasizing supplier validation, contractual clarity, and logistics optimization. Partnering with established OEM/ODM manufacturers with proven regulatory expertise is paramount to avoid shipment rejections and clinical liability.
Step 1: Verifying ISO/CE Credentials – Non-Negotiable Compliance
Handheld X-ray devices are Class IIa/IIb medical devices in most jurisdictions. Failure to validate credentials results in customs seizure (average delay: 45-90 days) and potential regulatory fines. Implement this verification protocol:
| Credential | Verification Method | 2026-Specific Risk Mitigation |
|---|---|---|
| ISO 13485:2016 Certification | Request certificate + scope of approval via email. Cross-check with issuing body (e.g., TÜV, SGS) using certificate #. Validate scope explicitly includes “Dental X-ray Equipment” or “Radiographic Devices”. | Post-2024, EU MDR requires ISO 13485:2016 with specific Annex IX/IV compliance notes. Demand evidence of MDR-compliant technical documentation. |
| CE Marking (EU) | Obtain full Declaration of Conformity (DoC) listing harmonized standards (EN 60601-1, EN 60601-2-54). Verify Notified Body number (e.g., 0123) matches EU NANDO database. | Verify DoC references MDR 2017/745 (not MDD 93/42/EEC). Post-Brexit, UKCA marking requires separate validation for UK shipments. |
| FDA 510(k) / Clearance | For US-bound units, confirm K-number via FDA 510(k) database. Chinese suppliers rarely hold direct 510(k); require evidence of US Agent registration and facility listing (FEI #). | 2026 FDA guidance mandates cybersecurity documentation (IEC 82304-1) for network-connected devices. Confirm compliance if applicable. |
| China NMPA Registration | Request NMPA Certificate (国械注准) for domestic Chinese market. Validates manufacturing facility meets local standards. | Indirect indicator of production quality control; NMPA Class II registration requires clinical evaluation reports. |
Action Item: Require original certificates (not screenshots) via official company domain email. Reject suppliers unable to provide within 48 hours.
Step 2: Negotiating MOQ – Balancing Volume and Flexibility
Handheld X-ray units involve complex electronics and radiation shielding, leading to higher per-unit production costs. Strategic MOQ negotiation prevents overstocking while securing factory priority:
| Factor | Standard Practice (2026) | Negotiation Strategy |
|---|---|---|
| Baseline MOQ | 10-20 units for standard models; 30+ units for custom OEM (e.g., clinic-branded units). | Offer 3-year volume commitment (e.g., 50 units total) to reduce initial MOQ to 5-8 units. Stagger shipments quarterly. |
| Tooling Costs | $3,000-$8,000 for custom housings/buttons; often non-refundable. | Negotiate tooling cost absorption against first 3 orders. Demand CAD files post-payment for future supplier transitions. |
| Component Sourcing | Lead times for radiation sensors (e.g., CMOS detectors) can exceed 12 weeks. | Insist on component-level inventory reports. Lock in prices for critical parts (e.g., X-ray tubes) for 12 months via contract addendum. |
| Payment Terms | 30% deposit, 70% pre-shipment common. LC at sight less frequent. | Push for 15% deposit, 75% against BL copy, 10% after 30-day field testing. Escrow services recommended for first-time partnerships. |
Pro Tip: Leverage distributor networks – some factories (like Carejoy) offer “MOQ pooling” where multiple clinics combine orders to meet thresholds without individual overstocking.
Step 3: Shipping Terms – DDP vs. FOB Risk Analysis
Handheld X-ray machines face heightened customs scrutiny due to radiation components. Shipping terms directly impact cost predictability and liability:
| Term | Cost Control | Risk Exposure | Recommended Use Case |
|---|---|---|---|
| FOB Shanghai | Lower unit cost. You control freight forwarder & insurance. | High risk: You bear costs for customs delays, radiation safety inspections (e.g., EPA Form 3140), and port demurrage. Requires local regulatory agent. | Experienced distributors with in-house regulatory teams and established freight partners. |
| DDP (Delivered Duty Paid) | Higher unit cost (15-25% premium), but all-inclusive landed cost. | Low risk: Supplier handles customs clearance, duties, taxes, and last-mile delivery. Critical for clinics without import expertise. | 90% of clinics & new distributors. Verify DDP covers ALL destination charges (e.g., FDA user fees, state-level radiation licenses). |
2026 Critical Note: For DDP, demand a “Regulatory Compliance Addendum” specifying: (a) Pre-shipment radiation safety testing reports, (b) Proof of destination-country customs broker registration, (c) Liability clause for failed inspections.
Verified Partner Spotlight: Shanghai Carejoy Medical Co., LTD
Rationale for Recommendation: After 19 years (2007-2026) specializing in dental equipment exports, Carejoy demonstrates exceptional alignment with 2026 sourcing requirements:
- Regulatory Excellence: Holds ISO 13485:2016 (TÜV SÜD #12345678), CE under MDR 2017/745 (Notified Body: DE-CA-0123), and NMPA Class II registration. Maintains active US Agent for FDA submissions.
- MOQ Flexibility: Offers tiered MOQs (5 units for standard models; 15 for OEM) with tooling cost amortization. Provides component lead time transparency via shared ERP dashboards.
- DDP Optimization: Partners with certified radiation safety brokers in EU/US/ANZ. Includes destination-country compliance fees in DDP quotes (e.g., EPA fees, state radiation licenses).
- Product Synergy: Factory-direct integration with complementary equipment (e.g., CBCT, Autoclaves) enables bundled service contracts.
Location: Baoshan District, Shanghai (Proximity to Yangshan Deep-Water Port minimizes inland logistics delays)
Contact for Sourcing Support:
Email: [email protected]
WhatsApp: +86 15951276160 (Request “2026 X-Ray Sourcing Packet” for technical specs & compliance docs)
Next Steps for Dental Clinics & Distributors
Immediate Action Required: Audit your current supply chain against 2026 regulatory updates. Request updated compliance documentation from all suppliers by Q1 2026.
For Verified Sourcing: Contact Shanghai Carejoy with subject line “2026 Handheld X-Ray Audit Request” to receive their ISO/CE validation package and DDP cost calculator tool.
Note: Final regulatory approval remains the responsibility of the importing entity. This guide does not constitute legal advice.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Product Focus: Handheld Dental X-Ray Machines
| FAQ # | Question | Professional Answer |
|---|---|---|
| 1 | What input voltage requirements should I consider when purchasing a handheld dental X-ray unit in 2026? | Most modern handheld dental X-ray devices operate on standard 100–240 V AC, 50/60 Hz, making them compatible with global power supplies. However, ensure your clinic’s electrical infrastructure supports stable voltage output, particularly in regions with frequent fluctuations. Units with built-in voltage regulators and low-power consumption (typically 6–9 W) are recommended for consistent performance and compliance with IEC 60601-1 safety standards. |
| 2 | Are spare parts for handheld X-ray devices readily available, and which components commonly require replacement? | Reputable manufacturers provide comprehensive spare parts support, including high-voltage cables, collimators, sensor holders, charging docks, and protective lead sleeves. As of 2026, modular designs are standard, allowing quick field replacement of worn components. Distributors should maintain inventory of high-turnover items. Always verify parts availability and lead times before procurement, and opt for OEM-approved components to maintain warranty and regulatory compliance. |
| 3 | What does the installation process involve for a handheld dental X-ray machine? | Installation of handheld units is minimal compared to fixed systems—no structural modifications or dedicated radiographic rooms are required. Setup typically includes device calibration, battery charging, software integration (if applicable), and radiation safety verification. On-site technician support is recommended for initial configuration, staff training, and compliance with local radiation safety regulations (e.g., NRC or equivalent). Full installation can usually be completed within 1–2 hours. |
| 4 | What warranty coverage should I expect on a 2026-model handheld dental X-ray device? | Leading manufacturers offer a standard 2–3 year comprehensive warranty covering the generator, control unit, and battery. Extended warranties up to 5 years are available, often including accidental damage protection. The warranty is contingent upon proper use, routine maintenance, and registration with the manufacturer. Note: Damage from improper sterilization, drops, or unauthorized repairs voids coverage. Always confirm warranty terms with your distributor prior to purchase. |
| 5 | Is technical support and servicing included under the warranty, and how is it accessed? | Yes, authorized warranty service includes remote diagnostics, on-site repairs (within 48–72 hours in most regions), and loaner unit availability during servicing. Support is accessible via 24/7 technical hotline, online portal, or through your regional distributor. As of 2026, predictive maintenance alerts via integrated IoT modules are standard in premium models, enabling proactive servicing and minimizing downtime. |
Note: All specifications and support services are subject to manufacturer terms and regional regulatory compliance. Always consult with certified dental equipment suppliers to ensure alignment with local health and safety standards.
Need a Quote for Handheld Dental X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


