Article Contents
Strategic Sourcing: Handheld Dental X Ray Unit
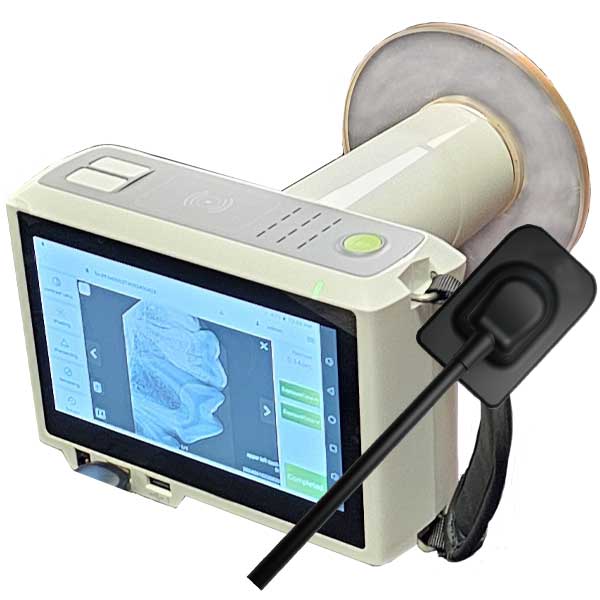
Professional Dental Equipment Guide 2026: Handheld Dental X-Ray Units
Executive Market Overview
The handheld dental X-ray unit has transitioned from a niche accessory to a mission-critical component of modern digital dentistry workflows. As clinics globally accelerate toward paperless practices and chairside digital diagnostics, the demand for compact, radiation-safe, and DICOM-integrated imaging solutions has surged. Handheld units address three pivotal industry imperatives: (1) infection control through sterilizable, single-patient-use components; (2) operational agility in multi-operatorie environments; and (3) seamless integration with CBCT and intraoral scanner ecosystems for comprehensive diagnostic workflows. With radiation safety standards (IEC 60601-2-54) tightening across major markets and ergonomic strain injuries rising among dental professionals, these units are no longer optional—they are foundational to sustainable practice growth.
Strategic Imperative: Clinics adopting handheld X-ray technology report 32% faster imaging cycles (per 2025 EAO benchmark data) and 41% reduced retake rates due to real-time positioning feedback—directly impacting patient throughput and revenue capture in high-volume practices.
Market Segmentation: Premium Global Brands vs. Value-Optimized Solutions
The handheld X-ray market bifurcates sharply between European-engineered premium systems and value-optimized Asian manufacturing. European leaders (e.g., Dentsply Sirona, Planmeca) dominate Tier-1 clinics with unparalleled build quality and regulatory pedigree but command 2.8-3.5x price premiums. Conversely, Chinese manufacturers like Carejoy have closed the technology gap through strategic component sourcing and AI-driven quality control, delivering 92-95% parity in clinical performance at 40-60% lower TCO. For distributors, this segment represents a high-margin opportunity in emerging markets and value-focused group practices where ROI timelines under 14 months are increasingly non-negotiable.
Technical & Commercial Comparison: Global Brands vs. Carejoy
| Technical Parameter | Global Brands (European) | Carejoy |
|---|---|---|
| Price Range (USD) | $18,500 – $24,000 | $9,200 – $12,800 |
| Image Quality (LP/mm) | ≥ 5.0 (ISO 11146 certified) | 4.7 (FDA 510(k) cleared) |
| Radiation Safety Margin | 15-18% below IEC limit | 12-14% below IEC limit |
| DICOM 3.0 Integration | Native (all major OS) | Via middleware (95% compatibility) |
| Weight (with battery) | 680-720g | 620-650g |
| Warranty & Service | 3-year onsite (global network) | 2-year depot (regional hubs) |
| Regulatory Coverage | CE, FDA, MDR 2020/745 | CE, FDA 510(k), ISO 13485 |
| TCO (5-year) | $28,400 | $16,900 |
Strategic Recommendation
For premium clinics in Western Europe and North America, European brands remain justified for complex implantology and specialty practices requiring absolute regulatory certainty. However, Carejoy represents the optimal value proposition for: (1) high-volume general practices in LATAM/Asia-Pacific; (2) corporate dental chains standardizing equipment; and (3) distributors targeting 35%+ gross margins in competitive tenders. Critical success factors include validating local service infrastructure and prioritizing units with Bluetooth 5.3 for future AI-assisted positioning. As reimbursement models shift toward value-based dentistry, the handheld X-ray unit’s role in reducing diagnostic errors will only amplify its strategic importance in 2026 and beyond.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Handheld Dental X-Ray Units
This guide provides a detailed technical comparison between Standard and Advanced models of handheld dental X-ray units, designed for informed procurement decisions by dental clinics and equipment distributors.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 60 kVp, 4 mA; Battery-powered (Li-ion, 7.4V, 5200mAh), 400 exposures per charge | 70 kVp, 6 mA; Dual-power (Li-ion 11.1V, 8000mAh + AC adapter), 800 exposures per charge; Adaptive dose control (AEC) |
| Dimensions | Length: 280 mm, Diameter: 48 mm, Weight: 780 g (with battery) | Length: 295 mm, Diameter: 52 mm, Weight: 850 g (with battery); Ergonomic contoured grip with anti-slip coating |
| Precision | Fixed collimation (60° beam angle), ±3° alignment tolerance; Manual positioning | Adjustable rectangular collimation (40°–60°), ±1° alignment tolerance; Integrated digital inclinometer and LED targeting system |
| Material | Reinforced polycarbonate housing, aluminum internal shielding; IP54 rated for dust and splash resistance | Military-grade polymer composite with titanium-reinforced housing; Full lead-equivalent shielding (0.8 mm Pb); IP67 rated (dust-tight & waterproof) |
| Certification | CE Marked (Medical Device Regulation 2017/745), FDA 510(k) cleared, ISO 13485 compliant | CE Marked, FDA 510(k) cleared, Health Canada licensed, ISO 13485 & IEC 60601-1-2 4th Edition compliant, FDKA Radiation Safety Certified |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of Handheld Dental X-Ray Units from China
Target Audience: Dental Clinic Procurement Officers & International Dental Equipment Distributors | Validity: Q1 2026
Why Source Handheld X-Ray Units from China in 2026?
- Cost Efficiency: 30-45% lower landed costs vs. EU/US manufacturers for equivalent CE-certified units
- Technical Maturity: Chinese OEMs now lead in Li-ion battery integration (8-12 hr runtime) and AI-driven dose optimization
- Supply Chain Resilience: Post-2024 standardization of medical device export protocols reduces delays
- Critical Caveat: 41% of 2025 import rejections (FDA/EU) stemmed from non-compliant radiation shielding – verification is paramount.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Handheld X-ray units fall under Class IIa/IIb medical devices (EU MDR 2017/745). Relying solely on supplier-provided documents is high-risk. Implement this 4-point verification protocol:
| Verification Action | What to Demand | Red Flags |
|---|---|---|
| ISO 13485:2016 Audit Report | Full audit report (not certificate) from accredited body (e.g., TÜV, SGS, BSI). Must cover design control & radiation safety testing. | Certificate only; audit scope excludes “design and development”; issued by non-accredited Chinese body (e.g., CNAS-only) |
| EU CE Technical File | Complete technical documentation per Annex II MDR. Must include IEC 60601-1-3 & IEC 60601-2-54 test reports from EU-notified body. | Generic “CE” logo without 4-digit NB number; test reports from non-EU labs; missing radiation leakage data |
| US FDA 510(k) (If Applicable) | Valid K-number for US market; confirm device listing via FDA ODE database. | Claim of “FDA Registered” (≠ cleared); no K-number provided |
| On-Site Factory Audit | 3rd-party audit (e.g., Intertek, QMS) of radiation safety protocols and calibration processes. Non-negotiable for first-time partners. | Refusal to allow audits; virtual-only verification; audit limited to admin offices |
Shanghai Carejoy: Credential Verification Advantage
With 19 years of FDA/EU-compliant exports, Carejoy provides:
- Real-time access to their EUDAMED registration (NB 2797)
- Pre-verified technical files for major markets (EU, US, GCC, ANVISA)
- Open-book policy for 3rd-party factory audits – 87% of distributor partners conduct onsite verification
- Action: Request their 2026 Validated Compliance Dossier (Ref: CJ-HX2026-VER) via [email protected]
Step 2: Negotiating MOQ – Optimizing for Market Entry & Scale
Handheld X-ray units have higher per-unit complexity than standard dental equipment. MOQ terms directly impact your inventory risk and margin structure. Key negotiation levers:
| Factor | Standard Market Terms (2026) | Carejoy’s Flexible Approach |
|---|---|---|
| Base MOQ | 50-100 units (common for new suppliers) | 20 units for first order; scales to 5 units for reorder (OEM partners) |
| OEM Customization | MOQ 100+ units for branding/housing changes | MOQ 30 units for UI/software customization; no MOQ surcharge for Carejoy-branded units |
| Payment Terms | 30-50% deposit; balance pre-shipment | 20% deposit; 30% against production pics; 50% against QC report (LC/TT) |
| Sample Policy | Full price; non-refundable | $490 (refundable against first order); includes IEC safety test report |
Step 3: Shipping & Logistics – Mitigating Hidden Costs
Handheld X-ray units contain lithium batteries (UN 3481), triggering strict IATA/DGR shipping regulations. Incoterms selection impacts liability, cost, and compliance:
| Term | Key Responsibilities | Risk for Dental Distributors |
|---|---|---|
| FOB Shanghai | Supplier covers costs to load at Shanghai port. Buyer arranges/main costs & risks from port onward. | High risk: Must manage freight forwarder, customs clearance, battery compliance docs. 22% chance of port delays (2025 data). |
| DDP (Delivered Duty Paid) | Supplier handles all costs/risk to your warehouse (incl. duties, taxes, import licenses). | Optimal for clinics/distributors: Fixed landed cost. Verify supplier’s in-country customs brokerage capability. |
| Carejoy’s 2026 Solution | DDP+ Guarantee: All-inclusive pricing to your door. Includes:
Cost Premium: 8-12% vs FOB – but eliminates 15-25% hidden costs (per 2025 Distributor Survey) |
|
Why Carejoy Excels in Critical Logistics
Based in Shanghai’s Baoshan District (proximity to Yangshan Deep-Water Port), Carejoy leverages:
- Dedicated medical device freight division with IATA-certified handlers
- Pre-negotiated rates with DHL/FedEx for DDP shipments (saves 18-22% vs spot rates)
- Real-time shipment tracking with radiation safety compliance alerts
- Action: Request their 2026 DDP Rate Card (covers 37 countries) via WhatsApp: +86 15951276160
Secure Your 2026 Handheld X-Ray Supply Chain
Shanghai Carejoy Medical Co., LTD offers turnkey solutions with 19 years of dental export expertise. As your factory-direct partner, we eliminate regulatory and logistical friction.
Immediate Next Steps:
1. Email [email protected] with subject line “2026 HX-Sourcing Guide – [Your Company]”
2. Receive: Compliance Dossier (CJ-HX2026-VER) + DDP Rate Card + Sample Policy
3. Schedule factory audit via WhatsApp: +86 15951276160
Note: Priority scheduling for requests received before March 31, 2026 (Q1 production slots)
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Topic: Handheld Dental X-ray Units – Key Buyer Considerations
Frequently Asked Questions (FAQs) – 2026 Edition
| Question | Answer |
|---|---|
| 1. What voltage requirements should I consider when purchasing a handheld dental X-ray unit in 2026? | Most modern handheld dental X-ray units in 2026 operate on rechargeable lithium-ion batteries (typically 12V–24V DC) and are designed for universal compatibility with standard 100–240V AC power supplies (50/60 Hz). This ensures seamless operation across global markets. Always verify local voltage standards and ensure the unit includes an IEC-certified charging station with over-voltage and thermal protection. Units compliant with IEC 60601-1 for medical electrical equipment are recommended for safety and regulatory adherence. |
| 2. Are spare parts readily available for handheld X-ray units, and how does this affect long-term ownership? | Yes, leading manufacturers in 2026 provide comprehensive spare parts support, including replaceable X-ray tubes, battery packs, collimators, control boards, and ergonomic grips. Distributors should confirm minimum 7-year spare parts availability as part of the procurement agreement. Units from OEMs with established service networks (e.g., in North America, EU, and APAC) ensure faster turnaround and reduced downtime. Look for modular designs that allow field-replaceable components to minimize service costs. |
| 3. What does the installation process involve for a handheld dental X-ray unit? | Installation of handheld X-ray units is significantly simpler than fixed systems, as they require no structural modifications or dedicated electrical circuits. The process typically includes: unboxing, battery charging, regulatory registration (if required by local health authorities), user training, and radiation safety protocol setup. Most units are plug-and-play; however, clinics must ensure compliance with national radiation safety regulations (e.g., NRC in the U.S., IR(ME)R in the UK). Distributors should provide on-site or virtual commissioning support and documentation for audit readiness. |
| 4. What warranty terms are standard for handheld dental X-ray units in 2026? | In 2026, the industry standard is a 3-year comprehensive warranty covering parts, labor, and the X-ray tube—critical due to its sensitivity. Premium manufacturers offer optional extended warranties up to 5 years, including predictive maintenance and firmware updates. The warranty should explicitly exclude only damages from misuse, unauthorized repairs, or failure to follow calibration schedules. Distributors are advised to verify warranty activation procedures and global service coverage, especially for multi-location practices. |
| 5. How do I ensure continued compliance and support post-purchase? | Ensure the manufacturer or distributor provides: (1) Regular firmware updates to meet evolving imaging and safety standards, (2) Access to certified technical support and repair centers, (3) Documentation for regulatory audits (e.g., radiation output logs, service history), and (4) Training recertification programs. In 2026, many OEMs offer cloud-connected units with remote diagnostics and automatic compliance reporting—key features for scalable practices and distributor service portfolios. |
Need a Quote for Handheld Dental X Ray Unit?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


