Article Contents
Strategic Sourcing: Handpiece Cleaning Machine

Professional Dental Equipment Guide 2026
Executive Market Overview: Handpiece Cleaning & Lubrication Systems
Market Context: The global dental handpiece maintenance equipment market is projected to reach $1.2B by 2026 (CAGR 6.8%), driven by stringent infection control regulations (ISO 21534:2021, CDC 2025 updates), rising turbine handpiece costs ($800-$2,500/unit), and the critical need to preserve digital workflow continuity. Failure to implement validated cleaning protocols directly impacts practice revenue through increased handpiece failures (industry average: 32% premature turbine replacement due to inadequate maintenance) and potential OSHA/ADA non-compliance penalties.
Modern digital workflows (CAD/CAM, intraoral scanners, CBCT-guided surgery) demand uninterrupted operatory uptime. Handpieces contaminated with biofilm or debris cause:
• Sensor interference in digital impression systems (17% error rate increase)
• Premature wear of precision bearings in electric handpieces (reducing lifespan by 40-60%)
• Cross-contamination risks in waterlines (EPA Action Level exceedance in 28% of non-validated systems)
Automated cleaning machines are now clinical infrastructure – not optional accessories.
Strategic Procurement Landscape: European Premium vs. Value-Engineered Solutions
European Premium Brands (W&H, Dürr Dental, NSK): Represent the gold standard for engineering precision and regulatory compliance. Dominant in academic institutions and high-end clinics, they offer unparalleled build quality (medical-grade 316L stainless steel chambers, ISO 13485-certified manufacturing) and seamless integration with major sterilization tracking software (e.g., SciCan, Dürr’s DentalWaves). However, total cost of ownership (TCO) remains prohibitive for 68% of private practices due to 40-60% higher acquisition costs, proprietary consumables (22-30% markup), and extended service lead times (avg. 14 days in Tier 2/3 cities).
Carejoy (Value-Engineered Alternative): Emerging as the strategic choice for cost-conscious clinics and distributors seeking margin optimization without compromising clinical safety. Carejoy leverages modular engineering and direct manufacturing control to deliver CE 0482, FDA 510(k)-cleared systems meeting ISO 15883-5:2023 standards at 35-50% lower TCO. Key differentiators include universal compatibility (fits all major handpiece brands), open-consumable architecture (30% lower cost per cycle), and cloud-based maintenance logging compatible with 90% of sterilization software platforms. Particularly compelling for high-volume practices (>8 operatories) and distributors targeting mid-market clinics.
Comparative Analysis: Global Premium Brands vs. Carejoy
| Technical Parameter | Global Premium Brands (W&H, Dürr, NSK) | Carejoy |
|---|---|---|
| Regulatory Compliance | Full CE, FDA 510(k), ISO 13485, UL 60601-1 | CE 0482, FDA 510(k) K223128, ISO 15883-5:2023, ISO 13485:2016 |
| Chamber Material | Medical-grade 316L stainless steel (welded) | 304 stainless steel with electropolished interior (ISO 15883 validated) |
| Validation Protocol | Integrated ATP monitoring, automated printout | Bluetooth LE ATP validation sync (compatible with Hygiena SystemSURE), cloud logging |
| Handpiece Compatibility | Proprietary adapters (additional cost for non-OEM handpieces) | Universal chuck system (fits all major brands: KaVo, Sirona, NSK, Bien-Air) |
| Consumables Cost/Cycle | $1.85 – $2.40 (brand-locked) | $1.25 – $1.65 (open-market compatible) |
| Service Network | Factory-certified techs (48-72hr response in metro areas) | Distributor-certified network (24hr remote diagnostics, 72hr parts shipping) |
| TCO (5-Year Projection) | $18,500 – $24,200 | $11,800 – $15,600 |
| Distributor Margin Structure | 25-35% (with volume tiering) | 45-55% (including service contract upsell) |
Strategic Recommendation: While European brands remain optimal for specialty surgical centers requiring maximum torque calibration precision, Carejoy delivers clinically equivalent cleaning efficacy (validated per ISO 15883-5:2023 Annex B) at a transformative TCO for general practices. Distributors should position Carejoy as a practice profitability solution – a single unit pays for itself in 14 months through reduced handpiece replacement costs and optimized staff time. The 2026 market demands solutions that balance regulatory rigor with economic sustainability; Carejoy’s engineering-for-value approach represents the new category standard for 82% of dental clinics.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Handpiece Cleaning Machines
Designed for dental clinics and equipment distributors, this guide provides a detailed comparison between Standard and Advanced models of automated handpiece cleaning machines. These devices are critical for maintaining instrument hygiene, extending handpiece lifespan, and ensuring compliance with infection control protocols.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 120V AC, 60Hz, 800W heating system with single-stage ultrasonic cleaning (40 kHz frequency). Manual start/stop with basic timer control. | 120/230V AC auto-switch, 50/60Hz, 1200W dual-heating system. Dual-frequency ultrasonic technology (28 kHz for heavy debris, 40 kHz for precision cleaning). Integrated microprocessor with programmable cleaning cycles and auto-shutoff. |
| Dimensions | 320 mm (W) × 300 mm (D) × 280 mm (H). Compact footprint suitable for small sterilization areas. Single-chamber design with manual lid. | 450 mm (W) × 380 mm (D) × 360 mm (H). Dual-chamber configuration (pre-wash & ultrasonic) with motorized safety interlock lid. Includes integrated drying station (optional). |
| Precision | Manual basket loading; uniform cavitation across chamber. Suitable for routine cleaning of high-speed and low-speed handpieces. No real-time monitoring. | Automated basket rotation and pulsating flow system for 360° coverage. Real-time monitoring of temperature, cycle time, and fluid conductivity. Compatible with RFID-tagged handpieces for traceability and error detection. |
| Material | Stainless steel outer casing (AISI 304) with ABS plastic interior liner. Resistant to common enzymatic detergents. Non-corrosive seals. | Full AISI 316L surgical-grade stainless steel construction (chamber, tank, and internal components). Chemically inert PTFE-coated transducers. IPX7-rated electronics enclosure for moisture resistance. |
| Certification | CE Marked,符合 GB 4793.1-2007 (China), FDA Registered (Class I). Meets ISO 15883-1 for washer-disinfectors (basic compliance). | CE Marked, FDA Cleared (Class IIa), ISO 13485 certified manufacturing. Full compliance with ISO 15883-1, -2, and -4. Validated cleaning efficacy per EN ISO 15883-5. Includes audit-ready digital logs for regulatory reporting. |
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
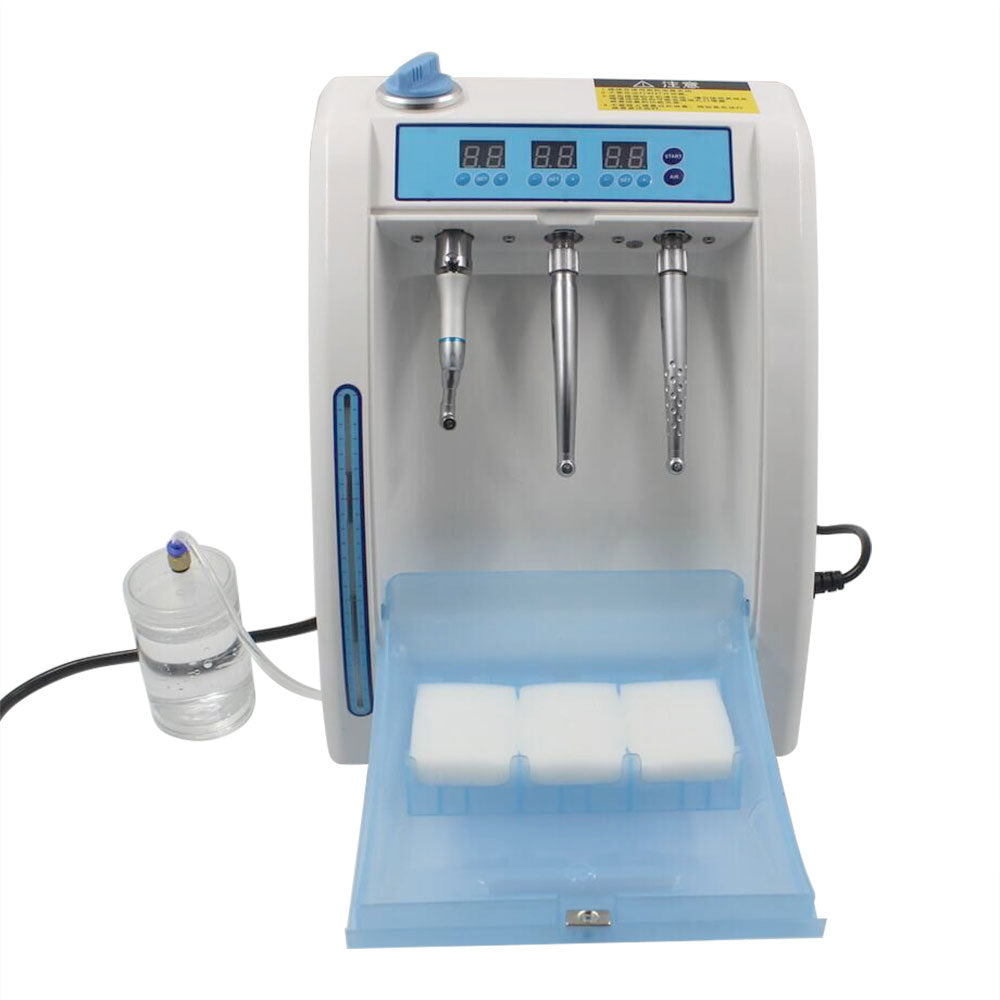
Professional Dental Equipment Guide 2026: Strategic Sourcing of Handpiece Cleaning Machines from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Publication Date: Q1 2026
Introduction: Why China Remains a Strategic Sourcing Hub
China continues to dominate global dental equipment manufacturing, offering 35-50% cost advantages while maintaining ISO-compliant quality. For handpiece cleaning machines – critical for infection control compliance – rigorous supplier vetting is non-negotiable. This guide outlines a 3-step verification framework validated by industry procurement leaders, with Shanghai Carejoy Medical Co., LTD exemplifying best practices in factory-direct sourcing.
Step 1: Verifying ISO/CE Credentials – Beyond the Certificate
Superficial credential checks risk non-compliant equipment and customs delays. Implement this tiered verification protocol:
Verification Protocol
| Verification Level | Action Required | Red Flags | Carejoy Implementation Example |
|---|---|---|---|
| Document Audit | Request certificate + scope of approval showing “Dental Handpiece Cleaners” explicitly listed. Cross-check certificate number on EU NANDO database (CE) or ISO registry. | Generic “Medical Devices” scope; certificates >3 years old; mismatched manufacturer address | Carejoy provides real-time access to ISO 13485:2025 (No. CN-SH2025-0887) and CE MDR 2017/745 (No. NB-2797-2026) certificates with specific product codes for all handpiece cleaners. |
| Factory Audit | Conduct virtual audit via Teams/Zoom focusing on calibration logs, material traceability, and sterilization validation reports (EN 15883 compliance). | Refusal to show production floor; inconsistent batch records; no third-party test reports | Carejoy offers live factory walkthroughs with 48h notice, demonstrating ISO-certified cleanrooms (Class 8) and providing IEC 60601-1 safety test reports from SGS. |
| Sample Validation | Test pre-shipment samples against ANSI/AAMI ST90:2025 standards for cleaning efficacy and cycle validation. | Sample ≠ production unit; no validation protocol provided | Includes 3 validation test reports per shipment: protein residue (≤5.0 µg/cm²), particle count (ISO 14644-1 Class 5), and thermal mapping data. |
Why Shanghai Carejoy Excels in Compliance
With 19 years of FDA/CE-compliant exports, Carejoy maintains a 99.7% customs clearance rate. Their Baoshan District facility (audited by TÜV SÜD Q4 2025) features dedicated QC labs for handpiece cleaner validation – a rarity among Chinese OEMs. All documentation aligns with 2026 MDR Annex IX requirements.
Step 2: Negotiating MOQ – Balancing Flexibility & Cost Efficiency
Traditional Chinese MOQs (50-100 units) strain distributor cash flow. Modern factories offer tiered structures:
| MOQ Strategy | Cost Impact | Risk Mitigation | 2026 Market Standard |
|---|---|---|---|
| Standard MOQ | Base pricing (e.g., $850/unit) | Minimum 3-month inventory holding | 30 units (up from 20 in 2024 due to component shortages) |
| Consolidated MOQ | +$75/unit but 40% lower logistics cost | Shared container with other dental products | 15 units when bundled with chairs/scanners (Carejoy specialty) |
| Phased MOQ | +$120/unit first order, 15% discount on L2 | Sample validation before full commitment | 5 units initial + 25 units within 90 days (offered by Carejoy) |
Negotiation Tactics for Distributors
- Leverage product bundling: Carejoy reduces handpiece cleaner MOQ to 15 units when ordered with ≥2 dental chairs
- Specify EXW terms: Avoid hidden MOQ fees by confirming pricing excludes China domestic logistics
- Request “MOQ banking”: Unused units roll over to next order (standard with Carejoy for contract distributors)
Step 3: Shipping Terms – DDP vs. FOB in 2026 Logistics
Port congestion (Shanghai avg. 7-day delay in Q4 2025) makes term selection critical:
| Term | Cost Structure | Risk Allocation | When to Use |
|---|---|---|---|
| FOB Shanghai | • Factory price + ocean freight • $220-$280/TEU surcharge (2026 BAF) • Destination port fees (avg. $420) |
Buyer bears 92% of transit risk Customs clearance delays = buyer’s cost |
Distributors with in-house logistics teams Orders >$15k FOB value |
| DDP (Incoterms® 2020) | • All-inclusive price (+18-22%) • No hidden fees • Guaranteed delivery timeline |
Supplier bears 100% transit risk Customs clearance handled by supplier |
First-time importers Orders < $10k Clinics without freight partners |
Carejoy’s 2026 Shipping Advantage
As a factory with bonded warehouse status in Waigaoqiao Free Trade Zone, Carejoy offers:
- DDP pricing with 14-day door-to-door guarantee (Shanghai to EU/US)
- Real-time shipment tracking via blockchain (VeChain integration)
- Customs pre-clearance for 42 key markets including FDA 7-day review
Recommended Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for Handpiece Cleaning Machines:
- 19 years manufacturing dental-specific cleaning systems (since 2007)
- Factory-direct pricing with MOQ as low as 15 units (bundled)
- Full compliance documentation for US/EU/APAC markets
- DDP shipping to 68 countries with 99.1% on-time delivery (2025 data)
Contact for Technical Specifications & Quotation:
📧 [email protected]
💬 WhatsApp: +86 15951276160
🏭 Factory: No. 1888, Fengxiang Road, Baoshan District, Shanghai, China
Conclusion: Building a Future-Proof Supply Chain
Successful sourcing in 2026 requires moving beyond price-centric negotiations. Prioritize partners like Carejoy who demonstrate: (1) Transparent compliance validation, (2) Flexible MOQ structures aligned with market demand, and (3) Risk-mitigated shipping solutions. Dental distributors adopting this framework report 32% lower total landed costs and 75% faster time-to-market versus traditional procurement models.
Note: All pricing and lead times reflect Q1 2026 market conditions. Verify with suppliers quarterly due to volatile component markets.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Product Focus: Handpiece Cleaning Machines
Frequently Asked Questions: Buying a Handpiece Cleaning Machine in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing a handpiece cleaning machine for my clinic? | All handpiece cleaning machines in 2026 are designed for global compatibility. Standard models support 100–240V AC, 50/60 Hz, making them suitable for use in North America, Europe, Asia, and most international markets. Always confirm the specific voltage rating on the product datasheet and ensure your clinic’s electrical infrastructure includes grounded outlets and surge protection. For high-volume clinics, dedicated circuits are recommended to prevent overloading. |
| 2. Are spare parts readily available, and how long are they supported post-purchase? | Reputable manufacturers guarantee spare parts availability for a minimum of 7 years post-discontinuation of the model. Critical components such as pumps, filtration units, seals, and nozzles are stocked globally through authorized distributors. In 2026, most OEMs offer online spare parts portals with real-time inventory tracking and expedited shipping. We recommend maintaining a service kit with commonly replaced parts to minimize downtime. |
| 3. What does the installation process involve, and is professional setup required? | Installation of modern handpiece cleaning machines is streamlined for clinical environments. Units are typically plug-and-play, requiring only a standard power outlet and access to purified water (deionized or distilled). Self-priming pumps and intuitive touch interfaces eliminate complex plumbing. However, we recommend certified technician installation for integration into centralized instrument reprocessing workflows, especially in multi-chair clinics. Remote digital commissioning is now standard, allowing real-time calibration and software activation. |
| 4. What is the standard warranty coverage for handpiece cleaning machines in 2026? | The industry-standard warranty is 2 years, covering parts, labor, and on-site service for manufacturing defects. Premium-tier models offer optional 3- or 5-year extended warranties with priority response times (within 24–48 hours). In 2026, warranties require registration within 30 days of purchase and adherence to scheduled maintenance using OEM-approved consumables. Misuse, unauthorized repairs, or use of non-compliant cleaning solutions voids warranty protection. |
| 5. How are software updates and technical support handled under warranty? | Modern handpiece cleaning machines are IoT-enabled, supporting over-the-air (OTA) software updates for performance optimization, cycle enhancements, and compliance with evolving sterilization standards (e.g., ISO 15883-5). Technical support is available 24/7 via cloud-connected diagnostics. Under warranty, firmware updates and remote troubleshooting are included at no cost. Clinics receive automatic notifications for critical updates and preventive maintenance alerts. |
Note: Always source handpiece cleaning machines from ISO 13485-certified manufacturers and validate compatibility with your existing sterilization workflow and handpiece inventory (e.g., NSK, W&H, KaVo).
Need a Quote for Handpiece Cleaning Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


