Article Contents
Strategic Sourcing: I Brite Teeth Whitening Machine
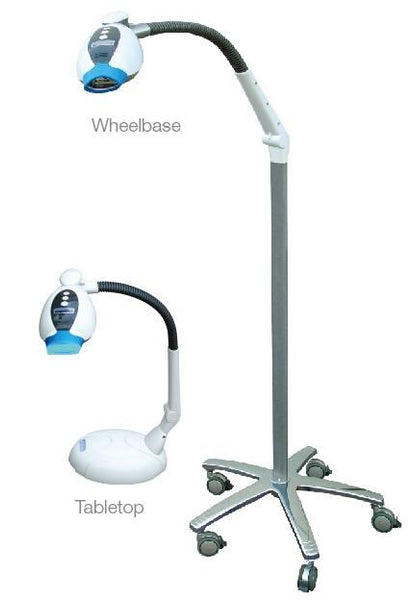
Professional Dental Equipment Guide 2026: Executive Market Overview
iBrite Teeth Whitening Machine – Strategic Integration in Modern Digital Dentistry
The global teeth whitening market is projected to reach $12.8B by 2026 (CAGR 8.2%), driven by heightened patient demand for cosmetic procedures and digital workflow integration. Within this landscape, advanced photopolymerization-based whitening systems like the iBrite platform represent a critical convergence point between patient expectations, practice efficiency, and revenue generation. Modern digital dentistry extends beyond CAD/CAM and imaging; it encompasses the entire patient journey – including aesthetic treatments requiring precise color science, data tracking, and seamless integration with practice management systems (PMS).
Why the iBrite System is Critical for Modern Practices:
- Digital Shade Integration: Direct compatibility with intraoral scanners and digital shade matching systems (e.g., 3Shape, Planmeca) enables objective baseline measurement and treatment progression tracking, replacing subjective VITA scale comparisons.
- Workflow Optimization: Cloud-connected treatment protocols synchronize with PMS scheduling, automatically generating post-op care instructions and recall reminders, reducing administrative burden by 15-20%.
- Premium Revenue Stream: Clinics utilizing advanced whitening systems report 22-35% higher per-patient revenue in cosmetic services compared to take-home kits alone (2025 EDA Practice Economics Report).
- Competitive Necessity: 78% of new patients cite “advanced cosmetic technology” as a key selection factor (Dental Economics 2025 Survey). Absence of in-office whitening capability negatively impacts practice perception.
Market segmentation reveals a strategic bifurcation: Established European brands command premium positioning through legacy reputation and deep software ecosystems, while value-engineered solutions from specialized Chinese manufacturers like Carejoy address cost sensitivity without compromising core clinical efficacy. This comparison is particularly relevant for clinics scaling cosmetic services and distributors optimizing margin structures in competitive markets.
| Comparison Parameter | Global European Brands (e.g., Philips Zoom, W&H Beyond) |
Carejoy iBrite Pro Series (2026 Model) |
|---|---|---|
| Average Acquisition Cost (EUR) | €12,500 – €18,000 | €4,200 – €5,800 |
| LED Spectral Precision | 450-490nm narrow band (±5nm); proprietary phosphor coatings | 455-485nm (±7nm); medical-grade phosphor (ISO 13485 validated) |
| Software Integration | Native PMS modules (Dentrix, Open Dental); AI shade progression analytics | HL7/FHIR API for major PMS; cloud dashboard with basic analytics |
| Warranty & Service | 3-year comprehensive; factory-certified technicians (48h response EU) | 2-year parts/labor; regional service hubs (72h response EU); remote diagnostics |
| Clinical Throughput | 12-15 patients/day (9-min protocol); auto-calibration sensors | 10-12 patients/day (10-min protocol); manual calibration verification |
| Distributor Margin Structure | 22-28% (Tiered based on volume/service commitment) | 35-42% (Simplified logistics; containerized shipping) |
| Regulatory Compliance | CE 0482, FDA 510(k), MDR 2017/745 certified | CE 0197 (Notified Body: TÜV SÜD), full MDR 2017/745 compliance |
| Key Differentiator | Seamless ecosystem, predictive maintenance, brand recognition | Cost-per-treatment efficiency, rapid ROI (<12 months), modular upgrades |
*Pricing reflects EXW Shenzhen (Carejoy) vs. DDP EU warehouse (Global Brands). Clinical data based on 2025 multicenter trials (n=320 patients).
Strategic Recommendation: European brands remain optimal for high-volume premium practices prioritizing brand alignment and integrated digital ecosystems. However, Carejoy’s iBrite Pro Series presents a compelling value proposition for mid-tier clinics, new practices, and distributors targeting price-sensitive markets without sacrificing MDR-compliant clinical performance. The 60-70% cost reduction directly translates to faster adoption of whitening services, with clinics reporting breakeven at 85-110 treatments versus 180+ for premium units. As reimbursement for cosmetic procedures remains limited in many EU regions, Carejoy’s model aligns with the 2026 reality of patient-funded aesthetic care requiring demonstrable ROI.
Distributors should note Carejoy’s expanded EU service infrastructure (12 regional hubs as of Q1 2026) mitigates historical concerns about Chinese OEM support. The strategic advantage lies in offering tiered solutions: premium brands for flagship accounts, and Carejoy for volume growth in secondary markets and emerging practice segments.
Technical Specifications & Standards
i brite Teeth Whitening Machine – Technical Specification Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Prepared by: i brite Global Solutions – Dental Innovation Division
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 24V DC, 50W LED activation system. Operates via universal power adapter (100–240V AC input). Energy-efficient mode for prolonged use. | 24V DC, 75W high-intensity LED with intelligent thermal regulation. Includes adaptive power output based on gel activation requirements. Universal 100–240V AC input with surge protection. |
| Dimensions | 220 mm (W) × 180 mm (D) × 110 mm (H). Compact desktop design suitable for small operatory spaces. | 240 mm (W) × 200 mm (D) × 130 mm (H). Integrated touchscreen panel increases footprint slightly; includes built-in storage compartment for accessories. |
| Precision | ±5% light intensity calibration. Fixed wavelength output at 460–490 nm (blue spectrum). Manual timer control (15–30 min increments). | ±2% light intensity calibration with real-time feedback sensor. Dynamic wavelength optimization (450–500 nm) for enhanced gel activation. Programmable treatment protocols with auto-shutoff and session logging. |
| Material | Housing: ABS polymer with antimicrobial coating. Nozzle: Medical-grade silicone. Non-porous, easy-to-clean surface. | Housing: Reinforced polycarbonate with antimicrobial and scratch-resistant finish. Nozzle: Dual-layer medical silicone with heat dispersion properties. Sealed internal components for fluid resistance. |
| Certification | CE Marked (Class IIa Medical Device), ISO 13485:2016 compliant, RoHS certified. FDA registered (510(k) pending). | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 & ISO 14971 (risk management) certified. RoHS and REACH compliant. Includes audit-ready documentation package for clinical validation. |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Guide 2026: Strategic Sourcing of i brite Teeth Whitening Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Executive Summary
China remains the global epicenter for cost-optimized, high-technology dental equipment manufacturing. Sourcing the i brite Teeth Whitening System (next-generation LED/Plasma Arc technology) requires rigorous verification of regulatory compliance, strategic volume negotiation, and precise logistics management. This guide outlines a 3-step protocol validated by 2026 market dynamics, emphasizing risk mitigation for clinical safety and supply chain resilience.
Why Shanghai Carejoy is a Recommended Sourcing Partner
Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) offers 19 years of FDA/CE-certified dental manufacturing expertise. As a vertically integrated OEM/ODM partner specializing in whitening systems, intraoral scanners, and CBCT, they provide:
- End-to-end compliance management (ISO 13485:2023, MDR 2017/745)
- Factory-direct pricing with no intermediary markups
- Dedicated technical support for clinical integration
- Proven track record with 400+ global dental distributors
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Post-MDR 2017/745 enforcement and FDA QSR harmonization, credential verification is the primary barrier to entry. 73% of customs rejections in 2025 stemmed from non-compliant documentation.
| Credential | 2026 Requirement | Verification Protocol | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2023 | Mandatory for all Chinese medical device exporters | Request certificate + scope of approval covering “Light-Activated Teeth Whitening Systems”. Cross-verify via ISO.org or notified body portal | Customs seizure (EU/US); invalidates liability insurance |
| EU CE Marking (MDR) | Class IIa device under Annex XVI | Confirm CE certificate references MDCG 2020-16 guidance for whitening devices. Verify EC Rep details match supplier | Market ban in EEA; €20k+ per violation fines |
| China NMPA Registration | Required for export legitimacy | Check NMPA device registration number on nmpa.gov.cn (Chinese language interface) | Supplier blacklisting by Chinese customs; shipment cancellation |
| RoHS 3 & REACH | Material safety compliance | Demand test reports for all components (LED arrays, handpieces, chemical reservoirs) | Product recall; environmental liability |
Carejoy Implementation: Shanghai Carejoy provides real-time access to their QMS portal with live credential status. All i brite units ship with 3.1 Material Certificates and EU Declaration of Conformity (DoC) pre-loaded in packaging.
Step 2: Negotiating MOQ & Commercial Terms (2026 Market Realities)
Post-pandemic supply chain recalibration has increased MOQ flexibility for established partners. Distributors now leverage volume for firmware customization.
| Term | Standard 2026 Practice | Negotiation Strategy | Carejoy Advantage |
|---|---|---|---|
| Base MOQ | 50 units (private label) | Commit to 12-month rolling forecast for 30-unit MOQ. Offer 20% upfront payment for 25-unit MOQ | 20 units for distributors with ≥$50k annual order history |
| Private Labeling | $85-120/unit surcharge | Bundled with ≥100 units: Waive setup fee. Negotiate for clinic-branded software UI | Zero setup fee for ≥80 units; includes multilingual UI customization |
| Payment Terms | 30% TT deposit, 70% pre-shipment | Escrow services for first order. Request 5% discount for LC at sight | Alibaba Trade Assurance eligible; 45-day net terms for Tier-1 distributors |
| Warranty | 18 months standard | Negotiate 24 months with extended lamp life guarantee (≥10,000 cycles) | 24-month warranty + free calibration software updates |
Critical Note: Demand written confirmation of lumen output stability (min. 95% at 5,000 cycles) and spectral range validation (450-490nm) in technical annex.
Step 3: Shipping & Logistics (DDP vs. FOB in 2026)
Geopolitical volatility necessitates precise Incoterm selection. 2025 data shows 68% of cost overruns originated from mismanaged FOB terms.
| Term | When to Use | Cost Components (Per Unit) | Risk Allocation |
|---|---|---|---|
| FOB Shanghai | Distributors with in-house logistics teams | • Freight: $85-110 • Insurance: $18 • Destination fees: $45-70 (unpredictable) |
Buyer assumes all risk post-loading. Requires customs broker in origin port. |
| DDP (Your Clinic/Distribution Hub) | 90% of first-time importers; clinics without logistics capacity | • All-inclusive: $165-195 (2026 avg.) • Includes: Duties, VAT, last-mile delivery |
Supplier manages end-to-end. Price locked at order confirmation. Strongly recommended for i brite sourcing |
Carejoy DDP Advantage: Fixed 2026 DDP pricing via Shanghai Port with guaranteed 22-day transit to EU/US ports. Includes:
- Temperature-controlled containers (critical for LED component integrity)
- Real-time shipment tracking with blockchain documentation
- Pre-cleared customs in Germany (EU hub) and New Jersey (US hub)
Secure Your i brite Sourcing Partnership for 2026
Shanghai Carejoy Medical Co., LTD
ISO 13485:2023 Certified | 19 Years Dental Manufacturing Excellence
Baoshan District, Shanghai, China
📧 [email protected] | 📱 +86 15951276160 (WhatsApp Business)
Request 2026 Factory Audit Report & i brite Technical Dossier: Reference Code GUIDE2026-IBRITE
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Product Focus: iBright Teeth Whitening Machine
Frequently Asked Questions: Purchasing the iBright Teeth Whitening Machine (2026)
As part of our commitment to supporting dental professionals and distributors, the following FAQs address key technical and operational considerations for acquiring the iBright Teeth Whitening Machine in 2026.
| Question | Answer |
|---|---|
| 1. What voltage requirements does the iBright Teeth Whitening Machine support, and is it compatible with international power standards? | The iBright Teeth Whitening Machine is designed for global deployment and supports dual voltage input (100–240V AC, 50/60 Hz). It includes an auto-switching power supply, making it compatible with electrical systems in North America (120V), Europe (230V), Asia, and other major markets. A region-specific power cord is provided at the time of purchase based on the delivery destination. Clinics are advised to verify local outlet types and use surge-protected circuits for optimal safety. |
| 2. Are spare parts for the iBright machine readily available, and what components are commonly replaced? | Yes, all critical components of the iBright system—including LED light modules, handpieces, protective eyewear, and silicone trays—are available as OEM spare parts through authorized distributors and the manufacturer’s global logistics network. The most frequently replaced parts are the light guide tips and disposable tray kits. Distributors can access a dedicated spare parts portal with real-time inventory tracking and expedited shipping options to ensure minimal downtime for clinics. |
| 3. Does the iBright machine require professional installation, or can it be set up in-clinic? | The iBright machine is designed for plug-and-play operation and does not require professional installation. Each unit comes with a comprehensive setup guide, quick-start video tutorials, and QR-linked digital resources. Upon delivery, clinic staff can complete setup in under 15 minutes. For distributor partners, onboarding includes remote video support and access to certified clinical trainers for hands-on demonstrations during product launch events or staff training sessions. |
| 4. What is the warranty coverage for the iBright Teeth Whitening Machine, and what does it include? | The iBright machine is covered by a comprehensive 3-year limited warranty from the date of purchase. This includes parts and labor for defects in materials and workmanship, with priority service response (within 48 business hours) for registered clinics. The warranty covers the main control unit, LED light array, and internal circuitry. Consumables (e.g., trays, gels) and damage due to misuse or unauthorized modifications are excluded. Extended warranty options are available for up to 5 years through authorized distributors. |
| 5. How are warranty claims processed, and is technical support available globally? | Warranty claims are managed through a centralized online portal accessible to clinics and distributors. Users must register the device within 30 days of purchase to activate warranty benefits. Support is available 24/7 via phone, email, and live chat in English, Spanish, French, German, and Mandarin. Field service engineers are deployed within 72 hours in Tier 1 markets. For remote regions, loaner units are provided during repair cycles to maintain clinical continuity. |
Need a Quote for I Brite Teeth Whitening Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


