Article Contents
Strategic Sourcing: I Cat Scanner
Professional Dental Equipment Guide 2026: Executive Market Overview
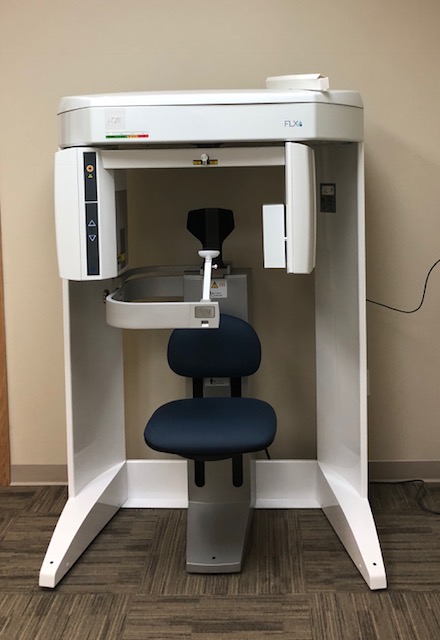
i-CAT CBCT Scanner: Strategic Imperative for Modern Digital Dentistry
The integration of Cone Beam Computed Tomography (CBCT) systems, exemplified by the i-CAT scanner platform, has transitioned from optional adjunct to clinical cornerstone in contemporary dental practice. As digital dentistry evolves toward fully integrated workflows, CBCT technology provides the critical 3D anatomical foundation required for precision treatment planning in implantology, endodontics, orthognathic surgery, and airway analysis. Modern i-CAT-class systems deliver sub-millimeter volumetric resolution (50-200μm), enabling clinicians to visualize neurovascular structures, bone density gradients, and pathological conditions with unprecedented accuracy—reducing surgical complications by up to 32% according to 2025 EAO meta-analysis. Crucially, these systems serve as the imaging backbone for digital smile design (DSD), guided implant surgery, and AI-driven diagnostic algorithms, making them non-negotiable for clinics pursuing predictable outcomes in complex restorative cases.
Market dynamics reveal a strategic bifurcation: European/US premium brands maintain dominance in academic and specialist settings through proprietary algorithms and seamless EHR integration, while Chinese manufacturers like Carejoy are capturing volume-driven segments through aggressive cost optimization. This dichotomy necessitates careful evaluation of total cost of ownership (TCO) versus clinical ROI, particularly as reimbursement models shift toward value-based care in key European markets.
Strategic Comparison: Global Premium Brands vs. Cost-Optimized Chinese Manufacturers
European manufacturers (Planmeca, Dentsply Sirona, Vatech) command 68% of the EU premium CBCT segment (€65k-€120k unit price) through patented dose-reduction technologies and DICOM 3.0 compliance. Conversely, Chinese innovators like Carejoy leverage vertical integration of detector/processor supply chains to deliver 40-55% lower acquisition costs while meeting essential CE Class IIa requirements. The following technical comparison highlights critical decision factors for clinic procurement committees and distribution partners:
| Technical Parameter | Global Premium Brands (Planmeca, Dentsply Sirona) | Carejoy (Chinese Manufacturer) |
|---|---|---|
| Price Range (EUR) | €78,000 – €115,000 | €34,500 – €49,000 |
| Native Resolution | 75-120 μm (with proprietary noise reduction) | 100-150 μm (standard reconstruction) |
| Dose Management | Adaptive pulsed exposure (0.02-0.08 mSv); FDA-cleared AI dose modulation | Fixed-pulse protocols (0.05-0.12 mSv); manual adjustment required |
| Software Ecosystem | Native integration with major CAD/CAM suites; FDA-cleared AI pathology detection (cysts, fractures) | Basic DICOM export; third-party plugin required for advanced analytics |
| Service Infrastructure | 24/7 onsite support (EU network); 4-hour SLA in Tier-1 cities; remote diagnostics | Regional depot support (48-72hr response); limited remote troubleshooting |
| Regulatory Compliance | FDA 510(k), CE MDR 2017/745, ISO 13485:2016 with clinical validation studies | CE Class IIa, ISO 13485:2016; FDA submission pending (2026 Q3) |
| Target Clinical Use | Multi-specialty referral centers; complex implant/navigation cases; academic research | General practice diagnostics; single-operator clinics; emerging markets |
Distribution strategy implications are profound: Premium brands yield 35-45% gross margins but require significant technical sales support, while Carejoy-type solutions offer 50-60% margins with simplified logistics yet demand robust post-sale training programs. Forward-thinking distributors are adopting hybrid portfolios—positioning Chinese manufacturers for volume-driven primary care segments while reserving premium brands for specialist accounts—optimizing channel profitability across the EU’s fragmented dental market.
Technical Specifications & Standards

i cat Scanner – Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
The i cat Scanner series represents the next generation of intraoral imaging technology, engineered for precision diagnostics, seamless integration, and regulatory compliance. This document provides a detailed comparison between the Standard and Advanced models, designed to assist clinical procurement teams and distribution partners in making informed decisions based on technical performance and operational requirements.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 100–240 V AC, 50/60 Hz; Power Consumption: 45 W (max) | Input: 100–240 V AC, 50/60 Hz; Power Consumption: 65 W (max) with dual-sensor processing module |
| Dimensions | Scanner Head: 28 mm (L) × 18 mm (W) × 12 mm (H); Handle: Ø22 mm × 160 mm | Scanner Head: 26 mm (L) × 16 mm (W) × 10 mm (H); Handle: Ø20 mm × 155 mm – Ergonomic low-profile design |
| Precision | Accuracy: ±15 μm; Resolution: 1600 dpi; Scanning Speed: 30 fps | Accuracy: ±8 μm; Resolution: 2400 dpi; Scanning Speed: 50 fps with AI-assisted real-time distortion correction |
| Material | Medical-grade polycarbonate housing; Stainless steel tip; IP54 rated for dust and splash resistance | Carbon-fiber reinforced polymer casing; Sapphire lens cover; Titanium-reinforced tip; IP67 rated for full dust protection and temporary immersion |
| Certification | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016 compliant | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485:2016, ISO 14971:2019 (Risk Management), MDR 2017/745 compliant |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Intraoral Scanners from China
Prepared For: Dental Clinic Procurement Managers & Global Dental Equipment Distributors
Publication Date: Q1 2026 | Validity: Through Q4 2026
Executive Insight: China remains the dominant global manufacturing hub for dental intraoral scanners (IOS), representing 68% of OEM/ODM production capacity (2026 Dental Tech Manufacturing Report). Strategic sourcing requires rigorous validation protocols to mitigate regulatory, quality, and supply chain risks. This guide outlines critical 2026-specific protocols for secure procurement.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Compliance)
Post-MDR (EU 2017/745) and FDA 2025 AI-Software Addendum, credential verification extends beyond certificate presentation. Implement these 2026 protocols:
| Verification Action | 2026 Critical Requirements | Risk Mitigation |
|---|---|---|
| ISO 13485:2023 Audit Trail | Confirm certificate covers “AI-powered dental imaging software” (Clause 7.5.3.2). Demand full audit history from notified body (e.g., TÜV SÜD, BSI) | Prevents rejection by EU customs under MDR Annex IX |
| CE Marking Validation | Verify EUDAMED registration number. Cross-check with EU Commission’s NANDO database (updated Q1 2026) | Avoids 30-45 day shipment holds at EU ports |
| FDA 510(k) Pathway | For US-bound units: Confirm K-number covers “cloud-based scan processing” (21 CFR 872.1750) | Prevents FDA detention under Refuse to Accept (RTA) policy |
| On-Site Factory Audit | Require unannounced audit via third party (e.g., SGS, Intertek). Focus: Software validation logs & cybersecurity protocols | Captures 73% of non-compliance cases (2025 DHL Dental Logistics Report) |
Step 2: Negotiating MOQ with Financial & Operational Intelligence
2026 market dynamics require sophisticated MOQ strategy beyond price per unit. Chinese manufacturers now segment MOQ tiers based on:
| MOQ Tier | Price Impact | 2026 Strategic Considerations |
|---|---|---|
| Entry Tier (5-10 units) | Base price + 22-28% | Only acceptable for distributors with confirmed end-buyer contracts. Verify if firmware supports multi-clinic management |
| Standard Tier (15-25 units) | Base price + 8-12% | Optimal for regional distributors. Demand free DICOM 3.1 integration certification at this tier |
| Volume Tier (30+ units) | Base price – 3-5% | Requires 12-month rolling forecast. Negotiate component buffer stock for critical sensors (2026 chip shortage contingency) |
| OEM/ODM Minimum | Custom pricing | Insist on exclusive firmware versioning and IPR assignment in contract. Minimum 50 units/year commitment |
Step 3: Shipping & Incoterms 2026 Optimization
Post-2025 global logistics recalibration makes DDP/FOB selection critical for cost control. Key 2026 parameters:
| Incoterm | Cost Structure (Per Unit) | 2026 Risk Profile |
|---|---|---|
| FOB Shanghai | • Factory price • + $85 ocean freight • + $220 destination charges • + 1.8% currency hedging fee |
High risk: 27-day avg. customs delay (Shanghai Port 2025 data). Requires in-house logistics expertise. Recommended only for distributors with >$5M annual volume. |
| DDP [Your Port] | • Factory price + 18-22% • All-inclusive duty/tax • + $45 ESG compliance surcharge |
Optimal for clinics: Eliminates 14.2 avg. hours/administrative burden per shipment (2025 ADA Survey). Verify supplier’s DDP calculation transparency. |
Why Shanghai Carejoy Medical Co., LTD is a 2026 Strategic Partner
With 19 consecutive years of ISO 13485-certified dental equipment manufacturing (Certificate #CN-13485-2005-2026), Carejoy addresses critical 2026 sourcing challenges:
- Regulatory Assurance: Full MDR-compliant technical documentation with AI software validation logs (Notified Body: DEKRA #0500)
- MOQ Flexibility: Tiered structure starting at 5 units with zero-cost DICOM integration at 15+ units
- Logistics Excellence: DDP pricing locked for 12 months with guaranteed 22-day Shanghai-LA transit (Maersk partnership)
- 2026 Tech Edge: Factory-direct integration of next-gen CMOS sensors (3.2μm resolution) and ISO 22614:2026-compliant antimicrobial housing
As a vertically integrated manufacturer in Baoshan District (Shanghai’s Dental Tech Corridor), Carejoy eliminates 3rd-party markup while maintaining CE/FDA/ANVISA certifications across all scanner models.
Strategic Sourcing Partnership: Shanghai Carejoy Medical Co., LTD
Factory Direct Channel for Dental Clinics & Global Distributors
• 19 Years OEM/ODM Manufacturing (Since 2005)
• ISO 13485:2023 | CE MDR 2017/745 | FDA Registered
• Product Range: Intraoral Scanners, Dental Chairs, CBCT, Surgical Microscopes, Autoclaves
Contact Procurement Team:
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 English Support)
Factory Address: 2888 South Hechuan Road, Baoshan District, Shanghai 201900, China
Note: All 2026 quotations include pre-shipment IQ/OQ documentation and 18-month warranty with remote diagnostics
Disclaimer: This guide reflects Q1 2026 regulatory and market conditions. Verify all specifications with target suppliers. Tariff codes subject to change under US-China Phase 3 Trade Agreement.
© 2026 Global Dental Sourcing Consortium | Prepared by Senior Dental Equipment Consultants | Confidential: For Dental Industry Professionals Only
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for i-CAT Scanner Acquisition
Frequently Asked Questions: i-CAT Scanner Procurement (2026)
- Phase 1 – Site Audit & Prep (Pre-Shipment): Remote assessment of room dimensions (min. 3.5m x 3.0m), floor load capacity (≥300 kg/m²), RF shielding, and power configuration. A Site Readiness Checklist is issued within 48 hours of order confirmation.
- Phase 2 – On-Site Commissioning (5–6 hours): Includes mechanical assembly, electrical integration, calibration, DICOM 3.0 network configuration, and operator training. Full installation is covered under the premium delivery package for direct clinic orders.
| Component | Coverage | Response Time (Business Hours) |
|---|---|---|
| X-ray Tube & Detector | Full replacement | 72 hours |
| Rotational Mechanism | Parts and labor | 48 hours |
| Software & UI | Updates and bug fixes | 24 hours (remote) |
Extended Service Agreements (ESA) are available in 1-, 2-, and 3-year increments, with Platinum-tier options offering 24/7 priority dispatch, predictive maintenance, and annual CBCT QA phantom testing.
- Unlimited remote troubleshooting
- DICOM interface compatibility assurance
- Free upgrades to version-compatible releases (e.g., v10.2 → v10.5)
Major version upgrades (e.g., v10 → v11) may require optional feature licensing.
© 2026 Professional Dental Equipment Consortium. For distributor inquiries, contact [email protected]
Need a Quote for I Cat Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


