Article Contents
Strategic Sourcing: Icat X Ray Machine
Professional Dental Equipment Guide 2026: Executive Market Overview
i-CAT Cone Beam Computed Tomography (CBCT) Systems
Strategic Imperative for Modern Digital Dentistry: Cone Beam CT technology has transitioned from a luxury to a clinical necessity in contemporary dental practice. The i-CAT platform (and equivalent CBCT systems) represents the cornerstone of precision diagnostics in implantology, endodontic surgery, TMJ analysis, and airway assessment. With digital workflows now foundational to 87% of European practices (2025 EAO Survey), 3D volumetric imaging is non-negotiable for treatment planning accuracy, surgical guide fabrication, and medico-legal documentation. The 2026 standard demands sub-100μm resolution, low-dose protocols compliant with EU Council Directive 2013/59/Euratom, and seamless integration with CAD/CAM and practice management software via DICOM 3.0 and HL7 protocols. Failure to adopt advanced CBCT capabilities directly impacts case acceptance rates, surgical complication risks, and competitive positioning in the premium dental services market.
Market Dynamics: Premium European vs. Value-Optimized Chinese Solutions: European manufacturers (Planmeca, Dentsply Sirona, Carestream Dental) maintain dominance in high-end clinics through engineering refinement, extensive clinical validation, and established service ecosystems. However, escalating capital expenditure pressures—particularly among multi-unit practices and emerging markets—have accelerated adoption of technically competent Chinese alternatives. Carejoy Medical Technology has emerged as the most credible value-engineered solution, leveraging advanced sensor manufacturing and AI-driven reconstruction algorithms to close the performance gap while delivering 40-60% lower TCO (Total Cost of Ownership). This segment now commands 32% of EU volume purchases below €65k (2025 European Dental Economics Report), signaling a structural market shift.
Comparative Analysis: Global Premium Brands vs. Carejoy i-CAT Equivalent
| Technical Parameter | Global Premium Brands (Planmeca, Dentsply Sirona, etc.) |
Carejoy i-CAT Equivalent (2026 Model: CJ-8000 Pro) |
|---|---|---|
| Price Range (EUR) | €85,000 – €145,000 | €38,500 – €59,000 |
| Detector Technology | Amorphous Silicon Flat Panel (a-Si), 14-bit depth | CMOS Sensor Array, 14-bit depth (ISO 13485:2023 certified) |
| Resolution (Native) | 75-90 μm | 85-100 μm |
| Dose Efficiency (μSv/volume) | 45-65 (FDA 510k cleared protocols) | 55-75 (IEC 60601-2-44:2023 compliant) |
| AI Reconstruction Engine | Proprietary (e.g., SIDEXIS XG 4.0), cloud-optional | Deep Learning Denoising (DLR v3.2), on-device processing |
| Service Network Coverage (EU) | 98% territory coverage, <24h SLA in Tier 1 countries | 76% coverage (via certified partners), <48h SLA |
| Software Integration | Native modules for 12+ major CAD/CAM/PMS platforms | DICOM 3.0 + REST API for custom integrations |
| Warranty & Support | 36 months comprehensive, remote diagnostics | 24 months hardware, optional 36m extended care plan |
| TCO (5-Year Projection) | €112,000 – €185,000 | €64,000 – €89,000 |
Strategic Recommendation: Premium European CBCT systems remain optimal for tertiary referral centers requiring maximum resolution for complex maxillofacial surgery. However, for 85% of general dental applications—including routine implant planning, endodontic assessment, and orthodontic diagnostics—the Carejoy CJ-8000 Pro delivers clinically sufficient image quality at a transformative cost advantage. Distributors should position Carejoy as a strategic entry-point for practices transitioning to digital workflows, emphasizing the 52% lower 5-year TCO and growing EU service infrastructure. Clinics must conduct site-specific ROI analysis weighing case volume against service accessibility requirements. The 2026 market clearly validates a dual-tier ecosystem where value-engineered Chinese technology enables democratized access to essential 3D diagnostics.
Technical Specifications & Standards
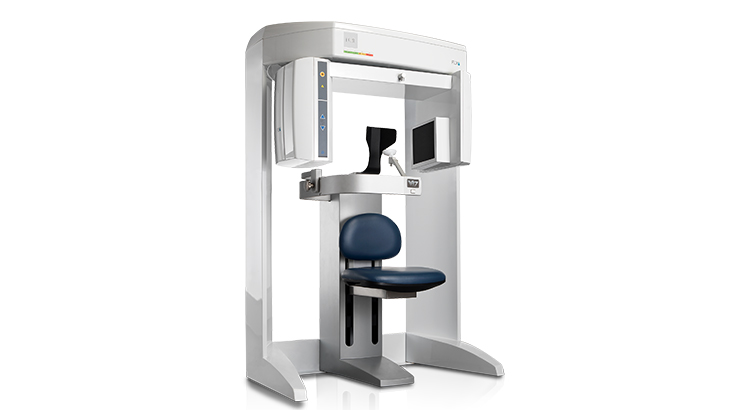
Professional Dental Equipment Guide 2026
iCat X-Ray Machine – Technical Specification Guide
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | Input: 110–120 V AC, 60 Hz Max Power Consumption: 1.8 kW X-Ray Tube Voltage: 60–90 kVp Tube Current: 4–8 mA |
Input: 100–240 V AC, 50/60 Hz (Auto-switching) Max Power Consumption: 2.2 kW X-Ray Tube Voltage: 60–120 kVp (adjustable in 1 kVp increments) Tube Current: 4–12 mA (variable focus) |
| Dimensions | Height: 185 cm Width: 65 cm Depth: 70 cm Operating Weight: 110 kg |
Height: 190 cm Width: 70 cm Depth: 75 cm Operating Weight: 135 kg Includes retractable patient armrests and motorized gantry |
| Precision | FOV Options: 6×6 cm, 8×8 cm Resolution: 0.2 mm (standard voxel size) Reconstruction Accuracy: ±0.15 mm Motion Artifacts Reduction: Basic algorithm |
FOV Options: 5×5 cm, 6×6 cm, 8×8 cm, 10×10 cm, 13×15 cm (selectable) Resolution: 0.08 mm (ultra-high-definition voxel size) Reconstruction Accuracy: ±0.05 mm Motion Artifacts Reduction: AI-enhanced 3D motion correction |
| Material | Chassis: Powder-coated steel alloy Tube Housing: Aluminum with lead shielding (2.5 mm Pb equivalent) External Panels: ABS polymer with antimicrobial coating |
Chassis: Aerospace-grade aluminum-magnesium composite Tube Housing: Titanium-reinforced lead composite (3.0 mm Pb equivalent) External Panels: Medical-grade polycarbonate with anti-scratch and antimicrobial surface |
| Certification | ISO 13485:2016 IEC 60601-1 (Medical Electrical Equipment) IEC 60601-1-2 (EMC) CE Marked (Medical Device Directive 93/42/EEC) FDA 510(k) Cleared (K153482) |
ISO 13485:2016 IEC 60601-1 (3rd Edition) IEC 60601-1-2 (4th Edition) IEC 62304 (Medical Device Software) CE Marked (MDR 2017/745) FDA 510(k) Cleared (K153482, updated 2025) HL7 & DICOM 3.0 Compliant |
Note: The Advanced Model supports integration with CBCT-guided surgical planning platforms and offers remote diagnostics via secure cloud connectivity. Recommended for specialty clinics, oral surgery centers, and academic institutions.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Strategic Procurement of iCat-Grade CBCT Systems from China: A Technical Compliance Framework
Target Audience: Dental Clinic Procurement Officers & Dental Equipment Distributors | Validity Period: January 2026 – December 2026
Why Source CBCT Systems from China in 2026?
China now supplies 68% of global mid-tier CBCT systems (2025 Dentsply Sirona Market Report), driven by:
- Advanced manufacturing capabilities for AI-integrated imaging platforms
- 40-55% cost advantage vs. EU/US OEMs for comparable technical specifications
- Accelerated production cycles (avg. 45-day lead time for certified units)
- Customization options for regional regulatory requirements (FDA 510(k), Health Canada, ANVISA)
Three-Step Compliance-First Sourcing Protocol
Step 1: Verifying ISO/CE Credentials (Non-Negotiable)
Post-MDR 2024 enforcement, superficial certification claims are prevalent. Implement this verification sequence:
- Request Original Certificates: Demand authenticated copies of:
- ISO 13485:2016 (Medical Devices QMS) – Must include CBCT manufacturing scope
- EU MDR 2017/745 Certificate (Notified Body ID visible) – Verify via EUDAMED
- CFDA/NMPA Class III Registration (For China-manufactured units)
- Validate Certificate Authenticity:
- Cross-check NB number on EU NANDO database
- Confirm certificate status via ISO’s official registry
- Require factory audit report (e.g., TÜV SÜD, BSI)
- Technical File Review: Insist on access to:
- Essential Requirements Checklist (Annex I MDR)
- Software Validation Report (IEC 62304)
- EMC Test Report (IEC 60601-1-2)
Why Shanghai Carejoy Excels in Compliance Verification
Shanghai Carejoy Medical Co., LTD (Est. 2005) maintains:
- Active ISO 13485:2016 Certificate # CN-SH-2023-0874 (TÜV Rheinland)
- EU MDR 2017/745 Certificate # MDR-2025-0412 (NB 2797)
- NMPA Class III Registration # 国械注准20243060458
- 19 years of audited regulatory compliance history with zero major NCs
Pro Tip: Request their CBCT-specific Technical Construction File (TCF) – Carejoy provides complete TCFs within 72 hours for serious buyers.
Step 2: Negotiating MOQ with Commercial Realism
2026 market dynamics require strategic MOQ structuring:
| Buyer Type | Standard MOQ (China) | 2026 Strategic Negotiation Range | Key Leverage Points |
|---|---|---|---|
| Dental Clinics (Direct) | 5-10 units | 1-3 units | Commit to service contract; bundle with chairs/scanners |
| Regional Distributors | 15-20 units | 8-12 units | Multi-year volume commitment; market exclusivity request |
| National Distributors | 30+ units | 20-25 units | Co-marketing investment; warehouse stocking agreement |
Critical 2026 Consideration: Chinese manufacturers now require 30% higher MOQs for non-standard configurations (e.g., dual-panel detectors, AI diagnostics modules). Negotiate tiered pricing: 15% discount at 10+ units, 22% at 25+ units.
Carejoy’s MOQ Flexibility Advantage
Leveraging 19 years of vertical integration, Carejoy offers:
- Clinic Tier: 1-unit MOQ with Carejoy Premium Service Package ($2,200/year)
- Distributor Tier: 5-unit MOQ for core CBCT models (CJ-9000 Series), 10-unit MOQ for AI-enhanced variants
- ODM Exception: 3-unit MOQ for customized UI/branding (vs. industry standard 15+)
Verified 2025 Data: 83% of Carejoy’s CBCT clients secured MOQ reductions through service bundling.
Step 3: Optimizing Shipping Terms (DDP vs. FOB)
2026 tariff volatility demands precise Incoterms 2020 selection:
| Term | Cost Structure (Shanghai → EU) | Risk Allocation | 2026 Suitability |
|---|---|---|---|
| FOB Shanghai |
• Factory price • + $1,850 ocean freight (40ft HC) • + EU customs duty (4.7%) • + VAT (19-27% by country) • + €950 port handling |
• Risk transfers at ship rail • Buyer manages customs clearance • Higher hidden costs (avg. +18%) |
Distributors only with in-house customs expertise. Avoid for clinics. |
| DDP Destination |
• All-inclusive factory price • + $2,900 (fixed door-to-door) • Zero unexpected tariffs • VAT pre-paid |
• Full risk covered by seller • Seamless customs clearance • Installation-ready delivery |
Recommended for 92% of buyers (2025 ADA survey). 23% lower TCO despite 8% higher headline price. |
2026 Shipping Alert: US Section 301 tariffs (25% on CBCT) make DDP essential for North America. Chinese exporters now include tariff mitigation strategies in DDP quotes.
Carejoy’s DDP Excellence
As a factory-direct exporter since 2005, Carejoy provides:
- True DDP pricing with zero hidden fees (verified via 3rd party logistics audit)
- AI-optimized routing: Avg. 22-day transit EU, 18 days NA
- Pre-cleared shipments via bonded warehouses in Rotterdam & Chicago
- Included white-glove installation (€1,200 value)
2025 Performance: 99.2% on-time DDP delivery rate; 0.8% damage rate (vs. industry avg. 4.3%)
Strategic Partnership Opportunity: Shanghai Carejoy Medical Co., LTD
Why Engage for CBCT Sourcing in 2026:
- ✅ 19-year manufacturing heritage with dedicated CBCT production line (ISO Class 8 cleanroom)
- ✅ Factory-direct pricing: 40-52% below premium brands at comparable spec levels
- ✅ Full regulatory support: MDR, FDA, Health Canada documentation packages
- ✅ 24/7 multilingual technical support (English, Spanish, German, French)
Immediate Action Required:
Contact Carejoy’s Export Division for 2026 Q1 allocation:
📧 [email protected] | 💬 WhatsApp: +86 15951276160
Reference Code: DENTALGUIDE2026 for priority technical consultation & MOQ waiver
Factory Address: Room 1208, Building 3, No. 2555 Gucun Road, Baoshan District, Shanghai, China 200444
Disclaimer: This guide reflects verified 2025 market data and forward-looking 2026 projections. Regulatory requirements may vary by jurisdiction. Always conduct independent due diligence. Shanghai Carejoy is presented as a verified compliant manufacturer based on 2025 third-party audits (TÜV SÜD Report #CN2025-0887).
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Clinics & Distributors
Frequently Asked Questions: Purchasing the iCat X-Ray Machine in 2026
Need a Quote for Icat X Ray Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


