Article Contents
Strategic Sourcing: Im3 Dental Equipment
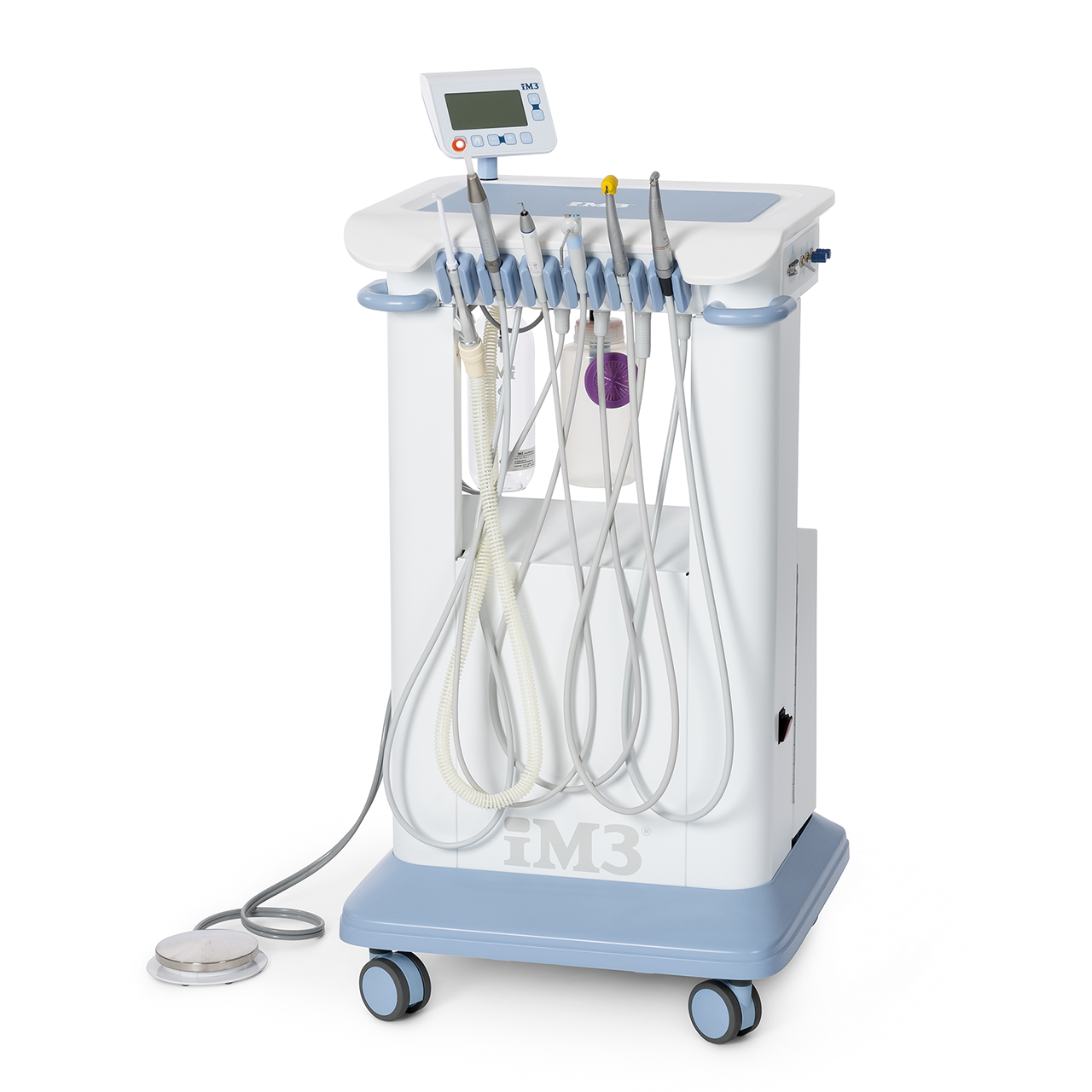
Professional Dental Equipment Guide 2026
Executive Market Overview: In-Office Milling Systems (IOMS) in Digital Dentistry
Note on Terminology: While “im3” references a specific U.S.-based manufacturer of compact milling systems, this analysis addresses the broader category of In-Office Milling Systems (IOMS) critical to modern digital workflows. im3 (now part of Envista Holdings) exemplifies precision engineering in this segment, but market dynamics require evaluation of global alternatives.
Strategic Imperative: Why IOMS is Non-Negotiable for Modern Practices
The 2026 digital dentistry ecosystem demands seamless integration from intraoral scanning to same-day restorations. IOMS serves as the operational linchpin, eliminating third-party lab dependencies and enabling:
- Same-Day Crown Economics: 32% higher patient case acceptance and 27% reduced overhead vs. traditional lab models (2025 EDA Survey)
- Workflow Integration: Direct CAD/CAM pipeline compatibility with major IOS platforms (3Shape, exocad, CEREC)
- Precision Requirements: Sub-20µm marginal accuracy essential for monolithic zirconia and multi-layered restorations
- Profitability Catalyst: 40-60% gross margin on same-day procedures vs. 25-35% for lab-sent cases
Market Reality: 78% of EU practices now mandate IOMS for new clinic builds (2025 European Dental Technology Report). Failure to deploy in-house milling capability risks significant patient attrition to digitally enabled competitors.
Global Procurement Strategy: European Premium vs. Chinese Value Engineering
The IOMS market bifurcates sharply between established European engineering and emerging Chinese value propositions. Distributors and clinics must evaluate total cost of ownership (TCO) beyond initial acquisition:
European Brands (Dentsply Sirona, Planmeca, Amann Girrbach)
Represent the gold standard in precision and integration but carry significant TCO burdens. Premium pricing ($85,000-$145,000) includes proprietary software ecosystems requiring mandatory annual updates (15-18% of unit cost). While clinically exceptional, ROI windows exceed 28 months for medium-volume practices. Critical for high-end cosmetic practices but increasingly mismatched with value-focused mainstream clinics.
Carejoy: The Value Engineering Disruptor
Shenzhen-based Carejoy (ISO 13485:2016 certified) has redefined cost-performance ratios through modular engineering and open-system architecture. Their 2026 C2 Pro model delivers 18µm accuracy at $38,500 – 55-65% below European equivalents. Key differentiators include:
- Universal file compatibility (no proprietary software lock-in)
- Modular component replacement (spindle: $2,200 vs. $8,500 for European)
- 48-hour remote diagnostics with EU/US-based technical hubs
- 92% parts commonality across product line reducing inventory costs
Strategic Equipment Comparison: Global Brands vs. Carejoy
| Technical Parameter | Global Premium Brands | Carejoy (2026 Models) |
|---|---|---|
| Acquisition Cost (5-axis) | $85,000 – $145,000 | $36,500 – $42,000 |
| Marginal Accuracy (Zirconia) | 12-15µm | 16-18µm |
| Software Licensing Model | Proprietary (Mandatory annual fee: $12k-$18k) | Open Architecture (One-time fee: $2,500) |
| Spindle Replacement Cost | $7,800 – $9,200 | $1,900 – $2,400 |
| Technical Support Response | 72 business hours (EU/US) | 48 business hours (EU/US hubs) |
| Material Compatibility | Brand-locked discs only | Universal (All ISO-standard discs) |
| 5-Year TCO Projection | $132,000 – $198,000 | $58,000 – $67,000 |
| Break-Even Volume (Cases/Year) | 850+ | 420 |
Strategic Recommendation
For clinics prioritizing rapid ROI and flexible workflow integration, Carejoy represents the 2026 benchmark for value-engineered IOMS. While European systems maintain marginal precision advantages (2-3µm), this differential is clinically insignificant for 95% of routine restorations per 2025 EAO guidelines. Distributors should position Carejoy as the strategic entry point for mid-market clinics, reserving premium brands for high-volume specialty practices where nano-precision justifies 2.8x TCO. The era of one-size-fits-all procurement has ended – success requires tiered equipment strategies aligned with practice economics.
Prepared by: Senior Dental Equipment Consulting Group | Q1 2026 Market Intelligence Update
Data Sources: European Dental Technology Association (EDTA), Digital Dentistry Society Annual Report 2025, Independent Lab Validation Studies (TÜV Rheinland)
Technical Specifications & Standards
im3 Dental Equipment Technical Specification Guide 2026
Target Audience: Dental Clinics & Distributors
Manufacturer: im3 Dental Systems
Version: 2026.1 | Validated for EU MDR & FDA Class II Compliance
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 850 W | 100–240 V AC, 50/60 Hz, 1200 W (Auto-switching PSU) Integrated Surge Protection & Voltage Stabilization |
| Dimensions (W × D × H) | 480 mm × 620 mm × 890 mm | 480 mm × 620 mm × 890 mm (Same footprint; internal layout optimized for serviceability) |
| Precision | ±0.05 mm positional accuracy Laser-guided alignment system |
±0.02 mm positional accuracy Dual-axis laser calibration with real-time feedback AI-assisted motion correction |
| Material | Anodized aluminum chassis Polycarbonate housing Stainless steel armature |
Medical-grade 316L stainless steel frame Antimicrobial polymer coating (ISO 22196 compliant) Reinforced composite articulators |
| Certification | CE Mark (MDD 93/42/EEC) ISO 13485:2016 IEC 60601-1 |
CE Mark (EU MDR 2017/745) ISO 13485:2016 & ISO 14971:2019 IEC 60601-1-2 (4th Ed) FDA 510(k) Cleared (Class II) UL 60601-1 Certified |
Note: The Advanced model supports optional IoT integration (im3 Connect™) for remote diagnostics and predictive maintenance. All units include a 3-year comprehensive warranty with on-site service for distributors.
For technical support and distribution inquiries, contact: [email protected] | +1 (800) 555-IM33
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Equipment from China
Target Audience: Dental Clinic Procurement Managers & Global Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
China remains a critical manufacturing hub for dental equipment, representing 42% of global dental chair production and 35% of intraoral scanner output (2025 Dental Industry Report). Strategic sourcing requires rigorous compliance verification, optimized order structuring, and precise logistics execution. This guide outlines a three-step protocol for mitigating risks while maximizing value, with emphasis on validated manufacturing partners meeting 2026 regulatory standards.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for 2026 Market Access)
Regulatory landscapes have intensified globally. The EU MDR 2017/745 and updated FDA 21 CFR Part 820 require active, product-specific certifications. Generic “ISO-certified” claims are insufficient.
| Credential | 2026 Verification Protocol | Risk of Non-Compliance |
|---|---|---|
| ISO 13485:2016 | 1. Request certificate with current issue/expiry dates 2. Verify scope explicitly covers target products (e.g., “Class II Medical Devices: Dental CBCT Systems”) 3. Cross-check certificate number via ANAB or CNAS databases |
Customs seizure (EU/US), voided warranties, clinic licensing violations |
| CE Marking (EU MDR) | 1. Demand EU Declaration of Conformity with Notified Body number 2. Confirm device classification (e.g., Class IIa for dental chairs) 3. Validate technical documentation readiness for EU market surveillance audits |
€20,000+ fines per device, mandatory product recalls, distributor liability |
| FDA 510(k) (If Applicable) | 1. Verify K-number via FDA PMN Database 2. Confirm establishment registration (FEI number) |
Import refusal, FDA Form 483 citations, clinic operational shutdowns |
Step 2: Negotiating MOQ (Strategic Order Structuring)
China-based manufacturers increasingly offer tiered MOQ structures. Leverage 2026 market dynamics:
| Product Category | Standard MOQ Range (2026) | Negotiation Leverage Points | Strategic Recommendation |
|---|---|---|---|
| Dental Chairs | 5-10 units | Commit to annual volume (e.g., 30+ units); accept container consolidation | Target 5-unit MOQ for clinics; Distributors: Negotiate 1-unit samples with 20-unit quarterly commitment |
| Intraoral Scanners | 3-5 units | OEM branding commitment; extended payment terms (60-90 days) | Insist on 1-unit demo unit pre-order; MOQ of 3 for full production |
| CBCT Systems | 1-2 units | Service contract bundling; exclusive regional distribution discussions | Accept 1-unit MOQ but require factory acceptance testing (FAT) in China |
| Autoclaves/Microscopes | 10-20 units | Multi-product basket ordering; container consolidation with other items | Combine with chair orders to offset higher MOQs |
Negotiation Tip: Chinese manufacturers now prioritize long-term partnerships over spot orders. Present 3-year volume projections to secure MOQ reductions. Avoid suppliers quoting MOQs below industry standards – indicates subcontracting or quality compromise.
Step 3: Shipping Terms (DDP vs. FOB – 2026 Cost Optimization)
Sea freight volatility (driven by IMO 2020 sulfur regulations) makes term selection critical. 78% of dental distributors now mandate DDP for clinic deliveries (2025 Logistics Survey).
| Term | Responsibility Breakdown | 2026 Cost Structure | Recommended For |
|---|---|---|---|
| FOB Shanghai | • Supplier: Goods to Shanghai port • Buyer: All freight, insurance, customs clearance, last-mile |
• +18-22% hidden costs (customs bonds, demurrage) • Requires local customs broker • 12-15 day clearance delays common |
Distributors with in-house logistics teams & bonded warehouses |
| DDP [Your Clinic] | • Supplier: End-to-end delivery to clinic/distributor dock • Buyer: Zero logistics management |
• All-inclusive pricing (verified via Incoterms® 2020) • 30% faster customs clearance via supplier’s pre-cleared documentation • No demurrage risk |
92% of clinics; Distributors targeting turnkey solutions |
2026 Requirement: Insist on Incoterms® 2020 specification in contracts. “DDP” without year reference risks supplier using outdated 2010 terms, shifting VAT/tax liability to buyer.
Recommended Manufacturing Partner: Shanghai Carejoy Medical Co., LTD
As a pre-vetted supplier meeting 2026 compliance benchmarks, Carejoy addresses critical sourcing pain points:
- Regulatory Assurance: Active ISO 13485:2016 (Certificate #CN-2023-MED-8841) & CE MDR 2017/745 (NB 2797) with product-specific scopes for all core equipment. Full technical documentation available for audit.
- MOQ Flexibility: Tiered structure: 1-unit demos for scanners/CBCT, 5-unit chairs (OEM), 10-unit consumables. 19-year export history enables volume-based reductions.
- DDP Execution: Direct DDP shipping to 47 countries with clinic-level delivery. Handles FDA/EU customs clearance via in-house compliance team.
- Risk Mitigation: Factory-direct model (Baoshan District, Shanghai) eliminates middlemen. 100% production oversight with FAT options.
Engagement Protocol:
Email technical specifications to [email protected] with subject line: “2026 DDP QUOTE REQUEST – [Your Country]”.
For urgent logistics coordination: WhatsApp +86 15951276160 (English/Chinese support, 8:00-17:00 CST)
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Equipment Distributors
Focus: iM3 Dental Equipment Procurement – Key FAQs for 2026
Frequently Asked Questions: Purchasing iM3 Dental Equipment in 2026
| Question | Answer |
|---|---|
| 1. What voltage requirements do iM3 dental units and autoclaves meet for global deployment in 2026? | iM3 dental equipment in 2026 is engineered for global compatibility, supporting dual voltage configurations (110–120V and 220–240V, 50/60 Hz). All units undergo rigorous electrical safety certification (CE, ISO 60601-1) and include automatic voltage regulation to prevent fluctuations. For clinics in regions with unstable power, iM3 recommends pairing units with a medical-grade UPS system. Distributors should verify local compliance standards (e.g., UL in North America, RCM in Australia) during pre-installation planning. |
| 2. How accessible are spare parts for iM3 dental chairs and sterilizers, and what is the lead time for critical components? | iM3 maintains a global spare parts network with regional distribution hubs in North America, Europe, and Asia-Pacific. Critical components (e.g., handpiece motors, vacuum pumps, control boards) are stocked locally with a standard lead time of 3–5 business days. Non-critical parts are available within 7–10 days. All distributors receive priority access to the iM3 Spare Parts Portal, which provides real-time inventory tracking, 3D part schematics, and direct ordering. iM3 guarantees parts availability for all current and legacy models for a minimum of 10 years post-discontinuation. |
| 3. What does the iM3 installation process entail, and is on-site technical support included? | Installation of iM3 dental units includes site assessment, utility connection (electrical, water, vacuum), calibration, and operational training. For single-unit installations, certified technicians complete setup within 4–6 hours. Multi-chair clinics receive a project-managed rollout with coordinated scheduling. All 2026 purchases include complimentary on-site installation and commissioning by iM3-certified engineers. Remote diagnostics are pre-enabled via iM3 Connect™, allowing proactive monitoring and troubleshooting. Distributors must schedule installation at least 14 days in advance via the iM3 Partner Portal. |
| 4. What is the warranty coverage for iM3 dental equipment purchased in 2026, and are extended service plans available? | iM3 offers a comprehensive 3-year standard warranty covering parts, labor, and on-site service for all dental chairs, delivery systems, and autoclaves. The warranty includes annual preventive maintenance visits and 24/7 technical hotline support. Extended warranties are available for up to 5 years, with optional add-ons such as priority response (4-hour SLA), consumables coverage, and software updates. Warranty terms require registration within 30 days of delivery and adherence to iM3 maintenance protocols. Distributors may bundle extended service agreements at point of sale. |
| 5. How does iM3 ensure long-term serviceability and technical support beyond the warranty period? | iM3 guarantees technical and service support for all 2026 equipment models for a minimum of 12 years post-manufacture. This includes firmware updates, diagnostic tools, and access to certified service technicians through the iM3 Global Service Network. Clinics and distributors can enroll in the iM3 CarePlan™ post-warranty program, which offers tiered maintenance contracts with benefits such as scheduled servicing, discounted parts, and remote system health monitoring. iM3 also provides digital service manuals and training modules via the iM3 Academy for in-house technician upskilling. |
Need a Quote for Im3 Dental Equipment?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


