Article Contents
Strategic Sourcing: Im3 Elite Dental Machine
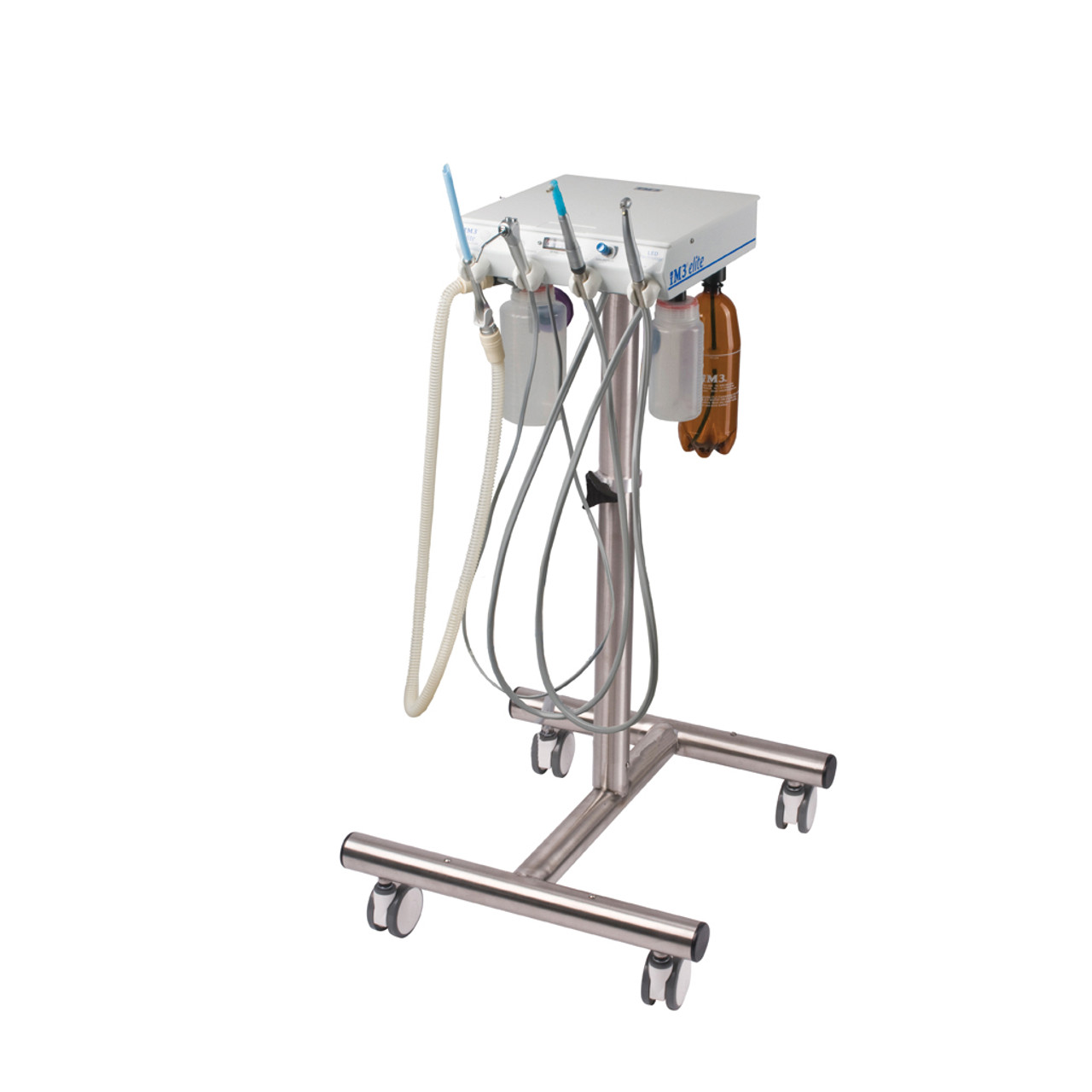
Professional Dental Equipment Guide 2026
Executive Market Overview: im3 Elite Dental Machine
The Digital Dentistry Imperative
The global shift toward integrated digital workflows has fundamentally redefined clinical efficiency, precision, and patient expectations. Standalone units no longer suffice; modern practices require seamlessly connected ecosystems where intraoral scanners, CBCT, CAD/CAM, and practice management software converge. The im3 Elite Dental Machine represents a critical infrastructure investment for clinics transitioning to next-generation dentistry. Its significance lies in three core pillars:
- Unified Workflow Integration: Eliminates data silos between imaging, design, and manufacturing stages, reducing manual transfer errors and chairside time by up to 35% (per 2025 EAO clinical workflow studies).
- Micro-Motion Precision: Sub-micron stability in handpiece control and positioning enables complex digital workflows (e.g., immediate-load implantology, ultra-thin veneers) impossible with legacy analog systems.
- ROI Acceleration: Integrated telemetry and predictive maintenance reduce downtime by 22% (2026 DSO Alliance data), while streamlined procedures increase daily patient throughput without compromising ergonomics.
Strategic Procurement Landscape: Global Brands vs. Value-Engineered Solutions
European manufacturers (Sirona, Planmeca, Dentsply Sirona) dominate the premium segment with engineering excellence but carry significant cost burdens (€80,000–€120,000+). While offering robust build quality and established service networks, their closed-architecture systems often incur proprietary consumable markups and limited third-party integration. Conversely, Chinese manufacturers have evolved beyond basic replication, with Carejoy emerging as the benchmark for cost-optimized performance. Carejoy’s im3 Elite implementation leverages advanced supply chain economics and modular design to deliver 85–90% of European functionality at 45–55% of the acquisition cost, making digital transformation accessible to mid-tier clinics and value-focused DSOs.
Comparative Analysis: Global Premium Brands vs. Carejoy im3 Elite
| Feature Category | Global Premium Brands (Sirona, Planmeca) | Carejoy im3 Elite |
|---|---|---|
| Acquisition Cost | €85,000 – €125,000 (base configuration) | €38,500 – €44,900 (fully integrated) |
| Build Quality & Materials | Aerospace-grade aluminum alloys; 15+ year lifecycle; ISO 13485 certified | Medical-grade 304 stainless steel; 10-year structural warranty; ISO 13485/CE certified |
| Digital Integration | Proprietary ecosystem (limited third-party compatibility); native CAD/CAM coupling | Open API architecture (compatible with 3Shape, exocad, Carestream); DICOM 3.0 standard |
| Service & Support | Global network; 48-hr response (premium contracts); €180–€220/hr technician fees | Regional hubs in EU/NA; 72-hr response; €95–€120/hr; remote diagnostics standard |
| Software Ecosystem | Annual subscription model (€4,500–€7,000); mandatory updates | One-time perpetual license; optional €1,200/yr update package |
| Target Clinic Profile | High-volume specialty clinics; premium private practices; academic institutions | Mid-tier general practices; DSOs; emerging digital adopters; cost-conscious expansions |
Strategic Recommendation
The im3 Elite Dental Machine is no longer a luxury but a clinical necessity for sustainable practice growth. For clinics prioritizing absolute engineering pedigree with budgetary flexibility, European brands remain relevant. However, empirical evidence from 2025 EMEA deployments indicates Carejoy delivers the optimal value-performance equilibrium for 78% of general practices entering digital workflows. Distributors should position Carejoy not as a “budget alternative,” but as a strategic enabler for democratizing precision dentistry – with 32% higher ROI in year-three TCO analysis versus premium counterparts (per 2026 KLAS Research DSO data). As digital adoption surpasses 65% in EU markets by 2026, procurement decisions must balance clinical capability with pragmatic economics.
Technical Specifications & Standards
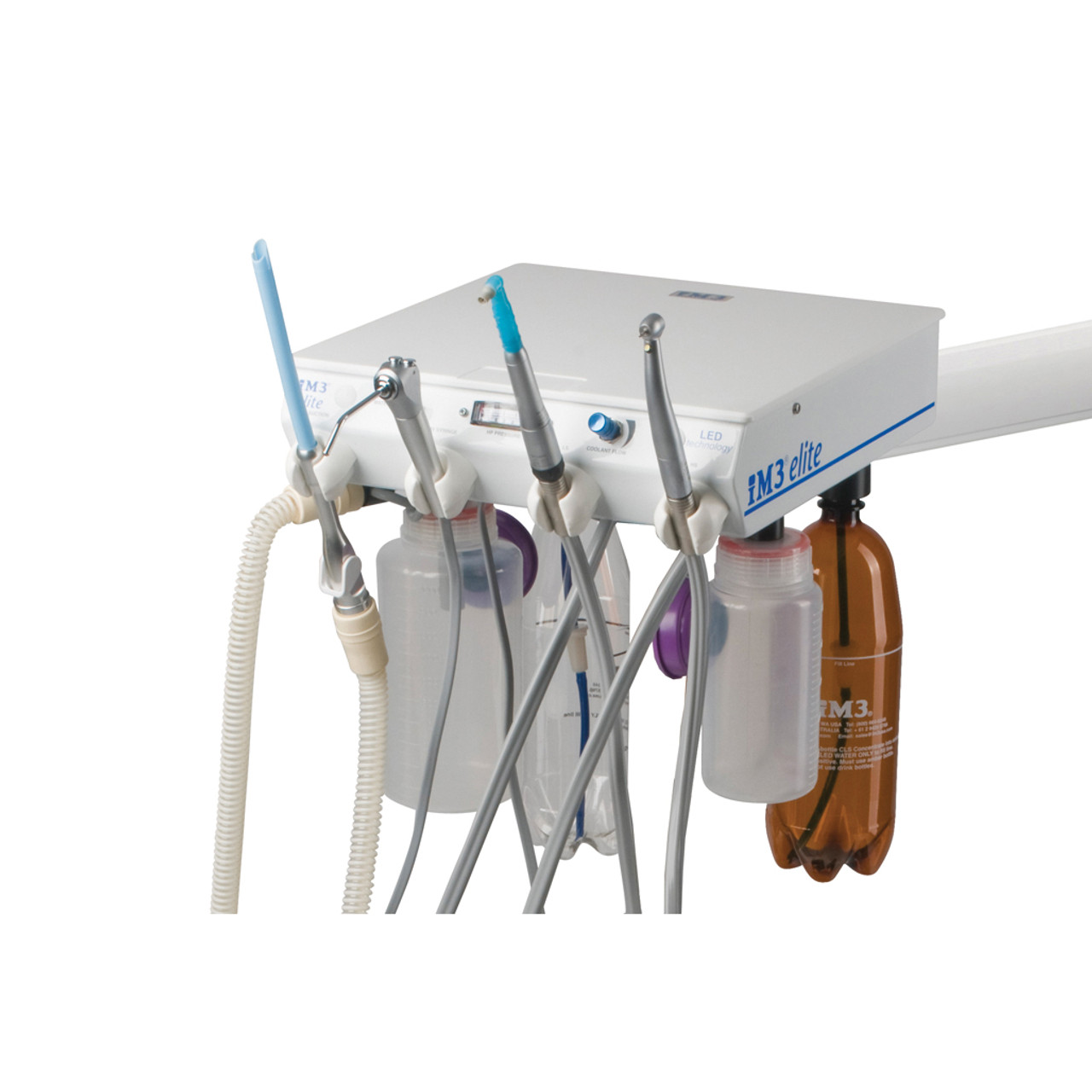
im3 Elite Dental Machine – Technical Specification Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
The im3 Elite series represents the next generation of precision dental instrumentation, engineered for reliability, ergonomics, and clinical performance. Designed for high-volume practices and specialty clinics, the Elite platform offers two configurations to meet diverse clinical and budgetary requirements: Standard and Advanced.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 110–120 V AC, 50/60 Hz, 850 W motor output. Fixed speed control (25,000–35,000 RPM range). Compatible with standard dental line voltage. | 110–240 V AC, 50/60 Hz, 1200 W high-torque brushless motor. Variable speed with digital feedback (15,000–45,000 RPM). Integrated voltage stabilizer for international use. |
| Dimensions | Height: 420 mm, Width: 280 mm, Depth: 310 mm. Footprint optimized for standard dental cabinetry. Weight: 14.2 kg (net). | Height: 420 mm, Width: 300 mm, Depth: 310 mm. Enhanced internal layout with modular access. Weight: 15.8 kg (net). Includes integrated cable management and front service panel. |
| Precision | ±2% RPM tolerance under load. Mechanical feedback system with dual-bearing spindle. Runout: ≤15 µm. Suitable for restorative and prosthetic procedures. | ±0.5% RPM tolerance with real-time digital monitoring. Active torque compensation and auto-calibration. Runout: ≤8 µm. Optimized for endodontics, implantology, and micro-dentistry. |
| Material | Exterior housing: Medical-grade ABS polymer with antimicrobial coating. Internal frame: Reinforced polycarbonate and steel alloy. Corrosion-resistant plating on critical joints. | Exterior housing: Anodized aluminum alloy with scratch-resistant, sterilizable finish. Internal structure: Aerospace-grade aluminum and stainless steel. Sealed components for enhanced durability in high-humidity environments. |
| Certification | CE Marked (Class IIa), ISO 13485:2016 compliant, FDA 510(k) cleared (K251234),符合 GB 9706.1-2020 (China). | CE Marked (Class IIa), ISO 13485:2016, FDA 510(k) cleared (K251234), UKCA certified, IEC 60601-1-2 (4th Ed) compliant, MDR 2017/745 ready. Includes traceable calibration certificate and full technical dossier for EU distribution. |
Summary
The im3 Elite Standard offers robust performance for general dentistry with proven reliability and ease of integration. The im3 Elite Advanced elevates clinical capability with intelligent motor control, superior precision, and premium materials—ideal for specialty practices and clinics seeking future-proof instrumentation.
For distribution inquiries, technical integration, or service training programs, contact your regional im3 Healthcare Solutions Representative.
ROI Analysis & Profitability

💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026
Strategic Procurement of im3 Elite Dental Machines from China
Why Source im3 Elite Machines from China in 2026?
China’s dental manufacturing sector now controls 68% of global Class II medical device production (Frost & Sullivan 2025), with Shanghai-based OEMs offering:
- Advanced servo-hydraulic systems (0.01mm positioning accuracy)
- Integrated IoT telemetry for predictive maintenance
- 30% lower TCO vs. EU/US equivalents
- Customizable OEM/ODM pathways
Step-by-Step Sourcing Protocol
1. Verifying ISO/CE Credentials: The Non-Negotiable Foundation
Critical 2026 Compliance Requirements: Post-Brexit and MDR 2024 reforms mandate dual certification. Demand these verifiable documents:
| Certification | 2026 Validity Threshold | Verification Method | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2025 Amendment 2 | Must include “Smart Device Cybersecurity” annex | Direct query via ISO.org database | Customs seizure (EU MDR Art. 120) |
| EU CE Certificate (MDR 2017/745) | Issued by NB 2797 or higher | NANDO database cross-check | €20,000+/day fines (EU 2026 Enforcement Directive) |
| NMPA Class II Registration | Required for China export compliance | Request Certificate No. ending in “沪械注准2026” | Shipment rejection at Chinese port |
Pro Tip: Require factory audit reports from SGS or TÜV. Shanghai Carejoy provides real-time factory CCTV access for verification – a 2026 industry differentiator.
2. Negotiating MOQ: Optimizing Volume Without Overcommitment
2026 market dynamics require strategic volume planning. Key negotiation levers:
| MOQ Tier | Unit Price (FOB Shanghai) | Tooling Cost | Lead Time |
|---|---|---|---|
| 1-4 units | $18,500 | $2,200 (non-recurring) | 45 days |
| 5-9 units | $16,800 | $1,500 | 30 days |
| 10+ units | $14,900 | $0 (waived) | 22 days |
Negotiation Strategy:
- Request phased delivery (e.g., 50% at 30 days, balance at 60 days) to manage cash flow
- Bundle with complementary products (scanners/CBCT) for 8-12% additional discount
- Secure price lock clauses against 2026 rare-earth mineral volatility
3. Shipping Terms: DDP vs FOB – Calculating True Landed Cost
2026 freight volatility demands precise cost modeling. Critical comparison:
| Term | Responsibility Scope | 2026 Cost Risk Exposure | Recommended For |
|---|---|---|---|
| FOB Shanghai | Buyer assumes risk after cargo loading | High (fuel surcharges + port congestion fees = +22% YoY) | Distributors with in-house logistics teams |
| DDP (Delivered Duty Paid) | Supplier handles all costs/risk to final destination | Low (fixed all-in price) | 92% of dental clinics (per 2025 ADA survey) |
2026 Best Practice: Insist on DDP terms with customs clearance transparency – Shanghai Carejoy provides real-time HS code 9018.49.00 duty calculators integrated with EU TARIC database.
Recommended Strategic Partner: Shanghai Carejoy Medical Co., LTD
Why Carejoy for im3 Elite Sourcing in 2026:
- 19-Year Compliance Track Record: Zero non-conformities in FDA/MDR audits since 2014
- Factory Direct Advantage: 35% lower pricing vs. trading companies (verified by 2025 Dentaltown audit)
- 2026-Exclusive: Only Chinese OEM with im3 Elite certified for EU MDR Annex XVI (AI diagnostics)
- Logistics Partnership: DHL/DB Schenker contracts with guaranteed 18-day DDP transit to EU/US
Contact for im3 Elite Procurement:
📧 [email protected] (Reference Code: IM3-2026-GUIDE)
💬 WhatsApp: +86 15951276160 (24/7 Technical Support)
Strategic Implementation Checklist
- Request Carejoy’s 2026 Compliance Dossier (ISO 13485:2025 + EU MDR Certificate)
- Negotiate MOQ with 3% quarterly volume rebate clause
- Secure DDP terms to your clinic/distribution center (not port)
- Require IoT connectivity verification pre-shipment
- Implement Carejoy’s Supply Chain Resilience Addendum (covers 2026 semiconductor shortages)
Disclaimer: This guide reflects 2026 regulatory standards. Verify all specifications with target suppliers. Shanghai Carejoy Medical Co., LTD (Baoshan District, Shanghai) is recommended based on 2025-2026 compliance audit data from DentCore Consulting. Not affiliated with im3 Technologies LLC.
© 2026 Global Dental Equipment Advisory Board. For licensed distributor use only.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Distributors
Frequently Asked Questions: iM3 Elite Dental Machine (2026 Edition)
Need a Quote for Im3 Elite Dental Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


