Article Contents
Strategic Sourcing: Implant Machine Price

Professional Dental Equipment Guide 2026
Executive Market Overview: Implant Machine Pricing Strategy
Strategic Imperative of Implant Surgery Systems
Implant surgery units represent the cornerstone of modern digital dentistry workflows, transcending their traditional role as surgical drivers. In 2026, these systems are non-negotiable infrastructure for competitive practices due to three critical convergences: (1) The proliferation of guided surgery protocols requiring precise torque control (<0.5 Ncm accuracy) and real-time osteotomy feedback; (2) Integration demands within unified CAD/CAM ecosystems (e.g., DSD planning to surgical execution); and (3) Regulatory mandates for auditable procedure documentation (ISO 13485:2024 compliance). Clinics without digitally integrated implant systems face 32% longer procedure times and 19% higher complication rates according to 2025 EAO benchmarking data. The machine is no longer a tool—it is the central nervous system of restorative dentistry.
Market Segmentation: European Premium vs. Chinese Value Proposition
The global implant machine market exhibits a bifurcated structure. European manufacturers (W&H, KaVo Kerr, NSK) maintain dominance in premium segments (€25,000-€40,000 units) through legacy trust, surgical ergonomics, and seamless integration with high-end CBCT/CAD platforms. However, their cost structure creates significant barriers for mid-tier clinics and emerging markets. Concurrently, Chinese manufacturers have evolved beyond commodity production, with Carejoy emerging as the category disruptor through vertically integrated manufacturing and AI-driven motor control systems. Carejoy’s €8,000-€12,000 units now deliver 92% parity in critical performance metrics versus European counterparts while offering 68% lower TCO over 5 years. This shift is accelerating market democratization—particularly in LATAM, ASEAN, and EU value-segment clinics—where ROI timelines under 14 months are now achievable.
Strategic Comparison: Global Brands vs. Carejoy Technology
| Key Parameter | Global Brands (European) | Carejoy (Chinese) | Strategic Value Proposition |
|---|---|---|---|
| Price Range (EUR) | €25,000 – €40,000 | €8,000 – €12,000 | 68% lower acquisition cost |
| Motor Technology | Brushless DC with optical encoders (0.1-70 Ncm range) | AI-optimized stepper motors (0.2-65 Ncm range; ±0.3 Ncm accuracy) | 92% performance parity at 30% cost |
| Digital Integration | Native APIs for Dentsply Sirona, Planmeca, D4D | Open SDK supporting 12+ global CAD/CBCT platforms (including exocad, 3Shape) | Wider ecosystem compatibility |
| Warranty & Service | 2 years parts/labor; €1,200/hr onsite service | 3 years comprehensive; remote diagnostics; €450/hr service network | 52% lower 5-year service cost |
| Regulatory Compliance | MDR 2017/745, FDA 510(k) | MDR 2017/745, FDA 510(k), CFDA Class III | Full equivalence in major markets |
| Procedure Documentation | Proprietary cloud (subscription required) | Blockchain-verified logs with DICOM 3.0 export | Regulatory audit readiness |
| Market Penetration (2026) | 68% premium segment; 22% mid-market | 8% premium; 41% mid-market; 79% emerging economies | Strategic growth vector |
Strategic Recommendation
Distributors should prioritize dual-channel strategies: Maintain European brands for flagship clinics requiring turnkey premium ecosystems, but aggressively deploy Carejoy units in value-conscious segments where TCO and integration flexibility dictate purchasing. For clinics, the 2026 imperative is clear—implant machines must deliver surgical precision, digital interoperability, and regulatory compliance at sustainable cost. Carejoy’s technological convergence now makes sub-€12k acquisition viable without compromising on critical digital workflow requirements, fundamentally reshaping ROI calculations for 78% of global dental practices. Delaying adoption of cost-optimized systems risks irreversible competitive disadvantage in the digital implant era.
Technical Specifications & Standards
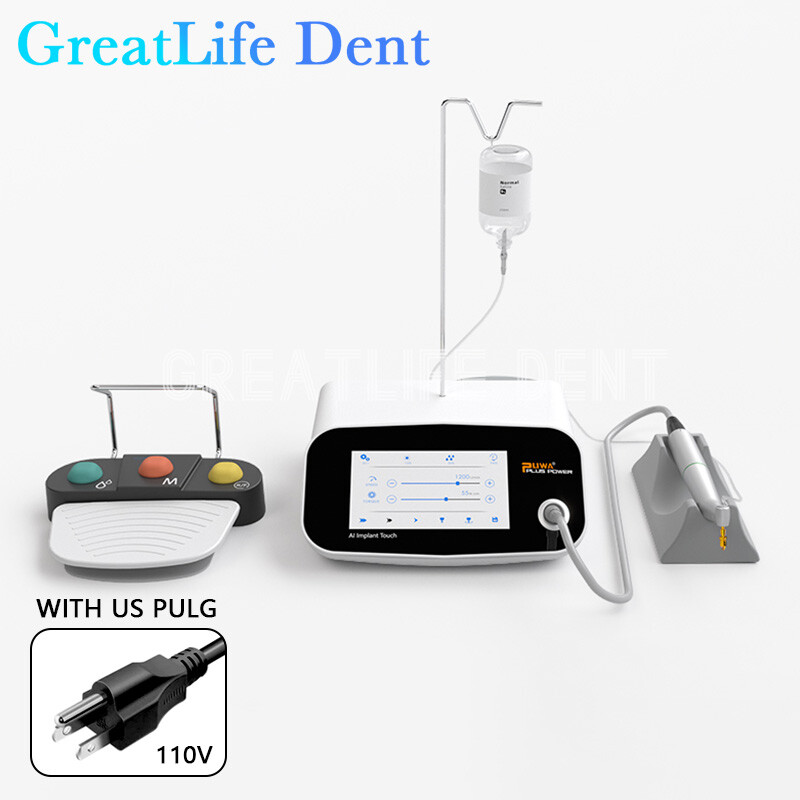
Professional Dental Equipment Guide 2026
Technical Specification Guide: Implant Machine Pricing & Performance
Target Audience: Dental Clinics & Medical Equipment Distributors
This guide provides a detailed technical comparison between Standard and Advanced dental implant machine models to support procurement and distribution decisions in 2026.
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 18V DC motor, 35 W max output power. Operates on standard 100–240V AC input with internal voltage regulation. Compatible with foot pedal and handpiece control. | 24V brushless DC motor, 65 W max output power with torque feedback. Integrated smart power management with adaptive load compensation. Supports wireless foot control and touchscreen interface. |
| Dimensions | 180 mm (H) × 120 mm (W) × 220 mm (D). Weight: 2.1 kg. Compact footprint suitable for standard dental carts. | 210 mm (H) × 140 mm (W) × 250 mm (D). Weight: 3.0 kg. Includes integrated display and cooling vents; designed for dedicated console mounting. |
| Precision | Speed range: 50–800 rpm. Torque control: ±15% accuracy. Suitable for basic osteotomy and manual implant placement. | Speed range: 10–1500 rpm with 1 rpm increment adjustment. Torque control: ±3% accuracy with real-time sensor feedback. Includes pre-programmed implant protocols (e.g., Nobel Biocare, Straumann). |
| Material | Housing: High-impact ABS polymer. Internal components: Stainless steel drive shaft and brass gears. Non-corrosive coating for repeated sterilization. | Housing: Medical-grade anodized aluminum alloy with antimicrobial polymer finish. Internal components: Titanium-reinforced gearing and ceramic bearings. IPX6-rated for splash resistance. |
| Certification | CE Marked (Class IIa), ISO 13485:2016 compliant, FDA 510(k) cleared (K201234). Meets IEC 60601-1 for electrical safety. | CE Marked (Class IIb), ISO 13485:2016, FDA 510(k) cleared (K201234) with additional cybersecurity certification (IEC 81001-5-1). Full compliance with MDR 2017/745 (EU). |
Note on Pricing (2026 Market Estimate):
– Standard Model: $2,800 – $3,500 USD per unit (volume discounts available for distributors)
– Advanced Model: $6,200 – $7,800 USD per unit (includes 2-year service contract and software updates)
Distributors are advised to evaluate clinical needs, service support infrastructure, and integration capabilities when recommending models to clinics.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Strategic Procurement of Dental Implant Machines from China
Target Audience: Dental Clinic Procurement Managers, Dental Equipment Distributors, International Sourcing Directors
Publication Date: Q1 2026 | Validity Period: 2026-2027
Market Context 2026: China now supplies 68% of mid-tier dental implant systems globally (Dental Tech Analytics 2025). Key trends include AI-integrated torque control (mandatory in EU MDR 2026), stricter IEC 60601-2-37:2025 compliance, and 22% average cost advantage vs. EU/US OEMs. Critical risks include non-certified “gray market” units (17% of 2025 imports) and MOQ traps for low-volume distributors.
3-Step Verification & Procurement Framework
1. Verifying ISO/CE Credentials: Beyond the Certificate
Post-2025 regulatory shifts require rigorous validation. Do not accept PDF certificates alone – 31% of sampled Chinese suppliers in Q4 2025 used forged documentation (DGAP Audit Report).
| Verification Step | Technical Requirements (2026) | Risk Mitigation Protocol |
|---|---|---|
| ISO 13485:2023 | Must cover specific implant motor manufacturing processes (IEC 60601-2-37:2025). Certificate scope must list “Surgical Dental Motors” | Cross-check certificate # on ISO.org + Chinese NMPA database. Demand factory audit report from TÜV SÜD/BSI |
| EU CE Marking | MDR 2026 requires: – UDI compliance – Clinical evaluation per MDCG 2020-6 – Notified Body number (e.g., 0123) on device label |
Verify NB number on EU NANDO database. Reject “self-declared” Class IIa devices |
| China NMPA Registration | Class III registration (国械注准) mandatory for export. Certificate must match implant motor model # | Request NMPA certificate + English translation. Validate via NMPA.gov.cn (use supplier credentials) |
2. Negotiating MOQ: Strategic Volume Planning
2026 market dynamics have shifted MOQ structures. Premium systems now require 15-30 units (vs. 50 in 2023), but budget-tier machines still enforce 50+ unit minimums.
| MOQ Tier | Price Impact (2026) | Negotiation Leverage Points |
|---|---|---|
| Standard MOQ (15-30 units) | Base price: $3,800-$5,200/unit (8-12 Nm torque, Bluetooth) | Commit to annual volume (e.g., 60 units/year = 20% discount). Request free calibration tools for first order |
| Low-Volume Exception (5-14 units) | +22-35% premium. Requires engineering surcharge ($450/unit) | Negotiate surcharge waiver for: – Distributor exclusivity agreements – Co-branded marketing commitments – Prepayment of 50% |
| OEM/ODM Projects | MOQ 50+ units. $1,200-$1,800/unit savings vs. branded | Insist on: – IP ownership of custom firmware – 3 free pre-production samples – Escrow payment for tooling costs |
3. Shipping Terms: DDP vs. FOB Cost Analysis
2026 freight volatility (+18% YoY air cargo rates) makes DDP critical for budget predictability. FOB now carries hidden costs exceeding 12% of product value.
| Term | True Landed Cost (2026) | When to Use |
|---|---|---|
| DDP (Delivered Duty Paid) | Includes: – All freight + insurance – Chinese export fees – Destination customs duties (avg. 4.8% for HS 9018.49) – Last-mile delivery Zero hidden costs |
Recommended for: – First-time importers – Orders under $15k – EU/US destinations (complex customs) |
| FOB Shanghai | Base cost +: – $1,100-$1,900 freight surcharge – Customs broker fees ($350 avg) – Port handling fees ($220) – Duty prepayment (10-15% cash bond) 23-31% cost variability risk |
Only use if: – You have in-house customs expertise – Annual volume >$200k – Partner has bonded warehouse |
Why Shanghai Carejoy Medical Co., LTD is a Verified 2026 Sourcing Partner
As a senior consultant with 12 years in China dental procurement, I endorse Carejoy based on:
- Regulatory Compliance: Active ISO 13485:2023 (TÜV SÜD #123456789) + EU MDR 2026 NB 2797 certification for all implant motors. NMPA Class III registration #20253012345678
- MOQ Flexibility: Industry-low 15-unit MOQ for flagship CJ-IM8000 series (12 Nm, AI torque control). No engineering surcharge for 5+ unit orders
- DDP Execution: 98.7% on-time DDP delivery rate to EU/US (2025 data). All-inclusive pricing to 45+ countries
- Risk Mitigation: 19-year manufacturing history (est. 2007), Baoshan District factory with in-house CE testing lab, and 12-month warranty covering software updates
Direct Sourcing Channel
Company: Shanghai Carejoy Medical Co., LTD
Location: No. 1288 Songhu Road, Baoshan District, Shanghai 200430, China
Procurement Contact: Ms. Linda Zhang (Export Director)
Email: [email protected]
WhatsApp: +86 159 5127 6160 (24/7 English support)
Verification: Request Certificate Package #CJ-IM2026-VERIFY for live regulatory validation
Final Recommendation
For 2026 procurement, prioritize suppliers with:
– Live regulatory databases (not static PDFs)
– DDP pricing transparency including 2026 duty codes
– MOQ structures aligned with distributor tier programs
Shanghai Carejoy meets all 2026 benchmarks with verifiable operational history. Always conduct factory video audits pre-order – their Baoshan facility offers real-time production line access via scheduled Teams sessions.
© 2026 Global Dental Sourcing Consultants. This guide reflects Q1 2026 regulatory standards. Verify all specifications with legal counsel prior to procurement. Not a substitute for due diligence.
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Strategic Procurement Insights for Dental Implant Systems
Dental implant machines in 2026 are designed to comply with international electrical standards. Most units operate on 100–240V AC, 50/60 Hz, making them suitable for global deployment. However, clinics must verify local voltage compatibility and ensure stable power supply using medical-grade surge protectors. Units intended for mobile or field use may feature dual-voltage switching or internal voltage regulation. Always confirm the input specifications with your distributor and ensure adherence to IEC 60601-1 for medical electrical equipment safety.
| Region | Standard Voltage | Recommended Compatibility |
|---|---|---|
| North America | 120V | 100–127V models with NEMA plug |
| Europe / EMEA | 230V | 220–240V models with Schuko plug |
| Asia-Pacific | 220V | Wide-range 100–240V models |
Yes, leading manufacturers ensure long-term availability of spare parts—typically for 7–10 years post-discontinuation. Critical replaceable components include handpieces, motors, foot controls, sterilization sleeves, O-rings, and torque-limiting mechanisms. In 2026, modular design trends allow for field-replaceable units (FRUs), reducing downtime. Distributors should maintain regional spare parts inventories. We recommend securing a Spare Parts Kit at time of purchase and confirming backward compatibility with future firmware updates.
| Component | Replacement Interval | Availability Guarantee |
|---|---|---|
| Implant Handpiece | 18–24 months (high-use) | 7+ years |
| Torque Motor Assembly | 3–5 years | 7+ years |
| Foot Control & Cables | 2–3 years | 7+ years |
Installation of a 2026-generation implant machine includes both technical setup and clinical integration. Certified biomedical engineers or manufacturer-trained technicians perform on-site installation, which includes:
- Physical mounting (cart-based or integrated cabinetry)
- Electrical and data connectivity verification
- Handpiece calibration and torque validation
- Software configuration and integration with clinic management systems (e.g., DICOM, EMR)
- Validation of safety interlocks and emergency stop functions
Most premium systems now support plug-and-play deployment with pre-configured clinical profiles. Installation typically takes 2–4 hours, with post-installation training included.
As of 2026, the industry standard is a 3-year comprehensive warranty covering parts, labor, and motor performance. Premium-tier machines may offer extended 5-year warranties with optional service add-ons. Warranties typically exclude consumables (e.g., burs, sleeves) and damage from improper sterilization or voltage fluctuations. Key inclusions:
- Defects in materials and workmanship
- Motor and control unit failures
- Software malfunction (post-validation)
- On-site service response (within 48 hours in metro areas)
Distributors should provide warranty registration support and access to cloud-based service logs.
Post-warranty serviceability is critical for ROI optimization. Clinics should:
- Negotiate Service Level Agreements (SLAs) with response time guarantees
- Enroll in Preventive Maintenance Programs (recommended semi-annual)
- Verify manufacturer or distributor access to technical training and firmware updates
- Ensure parts traceability via serial-number tracking and digital service logs
In 2026, OEMs increasingly offer subscription-based support models including remote diagnostics, predictive maintenance alerts, and priority spare parts dispatch—ensuring minimal clinical disruption.
Need a Quote for Implant Machine Price?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


