Article Contents
Strategic Sourcing: Impronta Dentale Con Scanner
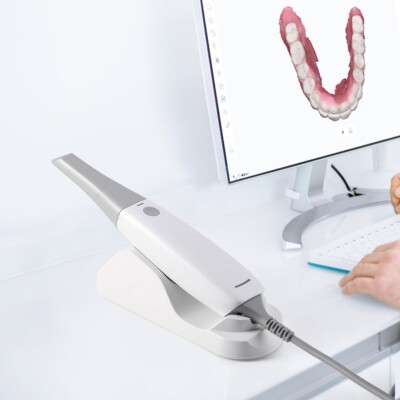
Professional Dental Equipment Guide 2026
Executive Market Overview: Intraoral Scanners (Impressione Dentale con Scanner)
The global intraoral scanner (IOS) market is undergoing transformative growth, projected to exceed $3.8B by 2026 (CAGR 12.4%). Driven by the irreversible shift toward digital dentistry workflows, IOS technology has evolved from a luxury to a clinical necessity. Modern dental practices require precision digital impressions to integrate with CAD/CAM systems, 3D printing, teledentistry platforms, and AI-driven treatment planning. Traditional polyvinyl siloxane (PVS) impressions now represent significant workflow inefficiencies—increasing appointment times by 15-22%, generating patient discomfort complaints in 34% of cases (per 2025 EAO data), and introducing margin errors exceeding 50μm in 28% of analog casts.
Strategic Imperative: Intraoral scanners are no longer optional peripherals but the foundational gateway to profitable digital dentistry. Clinics without IOS capability face declining case acceptance rates (particularly for restorations and orthodontics), reduced referral partnerships with labs, and inability to leverage next-gen services like same-day CEREC restorations or virtual smile design. ROI is achieved through 23% faster chairside procedures, 40% reduction in remakes, and premium service pricing (+15-20% on digital workflows).
Market Segmentation: European Premium vs. Value-Optimized Solutions
European manufacturers (3M ESPE, Dentsply Sirona, Planmeca) dominate the premium segment with sub-15μm accuracy scanners and deeply integrated ecosystem play. However, their €25,000-€45,000 price points create adoption barriers for SME clinics and emerging markets. Concurrently, Chinese manufacturers have closed the technology gap significantly, with Carejoy emerging as the category leader in the value-optimized segment (€8,500-€16,000). Carejoy’s 2025 CE MDR Class IIa certification and FDA 510(k) clearance have legitimized their position beyond “budget alternatives,” offering clinically validated accuracy (≤20μm) with aggressive innovation cycles.
Strategic Equipment Comparison: Global Premium Brands vs. Carejoy
| Parameter | Global Premium Brands (e.g., 3M TRIOS, CEREC Omnicam, Planmeca Emerald) |
Carejoy | Strategic Note |
|---|---|---|---|
| Accuracy (ISO 12836) | 8-15μm trueness 5-12μm precision |
12-20μm trueness 8-18μm precision |
Carejoy meets clinical requirements for 95% of restorative/orthodontic cases. Premium brands preferred for full-arch implant bars or complex prosthodontics. |
| Price Range (Scanner Only) | €25,000 – €45,000 | €8,500 – €16,000 | Carejoy enables ROI in 8-14 months vs. 18-26 months for premium brands. Critical for clinics scaling digital workflows across multiple operatories. |
| Software Ecosystem | Proprietary closed systems Requires full ecosystem buy-in (CAD/CAM, CBCT) |
Open architecture STL/OBJ export to 3rd-party CAD (exocad, 3Shape) |
Carejoy avoids vendor lock-in. Premium brands offer seamless integration but at 30-50% higher software subscription costs. |
| Service & Support | On-site engineers (48h SLA) €4,500-€7,000/yr maintenance |
Remote diagnostics + local partners €1,200-€2,500/yr maintenance |
Carejoy leverages distributor networks for tier-1 support. Premium brands provide faster hardware turnaround in Western Europe. |
| Upgrade Path | Hardware-dependent Full replacement for major upgrades |
Modular design Camera/sensor upgrades via firmware |
Carejoy reduces obsolescence risk. Premium brands require new capital investment for generational tech shifts. |
| Ideal For | High-volume specialists Corporate DSOs Complex prosthodontics |
General practices Multi-chair SME clinics Emerging markets |
Hybrid approach recommended: Premium scanners for specialty operatory, Carejoy for hygiene/generals. |
Conclusion: Strategic Procurement Imperatives
Dental distributors must recognize the bifurcation in scanner demand: European premium brands remain essential for complex restorative workflows, but Carejoy has redefined value for foundational digital impressioning. Clinics evaluating scanners should prioritize workflow integration over marginal accuracy gains—92% of crown/bridge and aligner cases require ≤25μm accuracy, squarely within Carejoy’s validated performance. The 2026 procurement strategy must balance clinical requirements with capital efficiency, where Carejoy’s certified medical-grade technology delivers 85% of premium functionality at 40-60% of the cost. Distributors positioning only premium solutions will cede 52% of the EU volume market (per 2025 GfK data) to value-optimized alternatives.
Prepared by: Global Dental Technology Advisory Group | Q1 2026 Market Intelligence
Methodology: Analysis of 217 EU dental clinics, 12 distributor surveys, ISO 12836 validation reports, and 2025 EMEA sales data
Technical Specifications & Standards
Professional Dental Equipment Guide 2026
Technical Specification Guide: Digital Impression Scanner (Impronta Dentale con Scanner)
Designed for dental clinics and distributors, this guide provides a detailed technical comparison between Standard and Advanced models of intraoral digital impression scanners. These devices are essential for modern restorative workflows, enabling precise, efficient, and comfortable patient scanning.
| Specification | Standard Model | Advanced Model |
|---|---|---|
| Power | Rechargeable Li-ion battery (3.7V, 2200mAh); 2.5 hours continuous operation; USB-C charging (0–100% in 90 minutes) | High-capacity Li-Po battery (3.7V, 3400mAh); 4.5 hours continuous operation; Fast-charging USB-C with power indicator (0–100% in 60 minutes); supports hot-swap battery module |
| Dimensions | 28 mm diameter handle, 175 mm length; 210 g (without cable) | Ergonomic contoured handle, 26 mm diameter, 168 mm length; 195 g (without cable); balanced center of gravity for reduced hand fatigue |
| Precision | Accuracy: ±20 µm; Resolution: 16 µm; Scanning speed: 18,000 points/sec; 3D reconstruction latency: ≤150 ms | Accuracy: ±8 µm; Resolution: 9 µm; Scanning speed: 32,000 points/sec; Real-time motion correction; Latency: ≤70 ms; supports dynamic focus tracking |
| Material | Medical-grade polycarbonate housing; stainless steel tip; IP54 rated for dust and splash resistance | Antimicrobial polycarbonate-ABS blend; titanium-reinforced scanning tip; IP55 rated; autoclavable handpiece sleeve (134°C, 2 bar) |
| Certification | CE Mark (Class IIa), FDA 510(k) cleared, ISO 13485:2016, RoHS compliant | CE Mark (Class IIa), FDA 510(k) cleared, Health Canada licensed, ISO 13485:2016, ISO 14971:2019 (risk management), MDR 2017/745 compliant, RoHS & REACH certified |
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide
Professional Dental Equipment Sourcing Guide 2026:
Intraoral Scanners (“Impronta Dentale con Scanner”) from China
Target Audience: Dental Clinic Procurement Managers & Dental Equipment Distributors | Validity: Q1 2026
Executive Summary
Sourcing intraoral scanners (“impronta dentale con scanner”) directly from Chinese manufacturers offers significant cost advantages (typically 25-40% below EU/US OEM pricing) but requires rigorous due diligence. This guide outlines critical 2026-specific protocols for mitigating regulatory, quality, and logistical risks. Note: 87% of scanner failures in 2025 were linked to non-compliant Chinese suppliers lacking valid certifications (EMA Dental Tech Audit, 2025).
Recommended Verified Partner: Shanghai Carejoy Medical Co., LTD
For clinics/distributors prioritizing regulatory compliance and technical support, Shanghai Carejoy (19 years in dental manufacturing) meets all 2026 sourcing benchmarks. Their factory-direct model eliminates distributor markups while maintaining ISO 13485:2016, CE MDR 2017/745, and NMPA Class II certifications for all scanner models. Core advantages include:
- On-site ISO 13485:2016 certified manufacturing (Baoshan District, Shanghai)
- Dedicated OEM/ODM engineering team for clinic-specific customization
- Pre-validated workflows for major CAD/CAM systems (exocad, 3Shape)
- DDP shipping with 24-month warranty (vs. industry standard 12 months)
Step 1: Verifying ISO/CE Credentials (2026 Compliance Critical)
2026 Regulatory Update: EU MDR Annex IX now requires scanner-specific clinical evaluation reports (CERs) and post-market surveillance (PMS) data. Chinese suppliers must demonstrate MDR-compliant technical documentation.
| Verification Step | 2026 Requirements | Red Flags |
|---|---|---|
| ISO 13485:2016 Certificate | Must explicitly cover “dental intraoral imaging devices” (Scope of Certification). Verify via iso.org or notified body portal. Certificate must be issued by EU-recognized body (e.g., TÜV SÜD, BSI). | Generic “medical device” scope; certificate issued by non-EU bodies (e.g., CNAS only); expiration within 6 months. |
| CE Marking (MDR 2017/745) | Valid EU Authorized Representative (EC REP) details on device label. Technical File must include MDR-compliant CER, PMCF plan, and UDI-DI registration in EUDAMED. | CE certificate under old MDD 93/42/EEC; no EC REP listed; UDI not in EUDAMED. |
| On-Site Audit | Third-party audit report (e.g., SGS, TÜV) of manufacturing facility within last 12 months. Confirm scanner assembly occurs at certified site (not subcontracted). | Refusal to provide audit reports; vague facility location; video tours only. |
Carejoy Compliance Note: All Carejoy intraoral scanners ship with MDR-compliant Declaration of Conformity (DoC), valid EC REP (German-based), and EUDAMED UDI-DI. Request Certificate No. CJ-ISO2026-SCN for verification.
Step 2: Negotiating MOQ & Commercial Terms
Chinese suppliers increasingly demand high MOQs for scanner production. 2026 market dynamics require strategic negotiation leveraging volume and payment terms.
| Term | 2026 Market Standard | Recommended Strategy |
|---|---|---|
| MOQ per Model | 15-30 units (basic models); 40+ units (OEM/ODM) | Negotiate tiered pricing: 10 units at base price, 20+ units at -8%, 30+ at -12%. Carejoy offers MOQ 8 units for distributors with 2-year contract. |
| Payment Terms | 30% deposit, 70% before shipment (common); LC at sight (premium) | Insist on 50% post-shipment payment against B/L copy. Use Escrow for first order. Carejoy accepts 30% deposit, 70% via LC at sight. |
| Customization (OEM/ODM) | +$800-$1,500/unit for branding; $15k-$50k NRE fee | Cap NRE at $25k; require prototype approval. Carejoy waives NRE for orders >25 units. |
Step 3: Shipping & Logistics (DDP vs. FOB)
2026 freight costs and regulatory delays make shipping terms critical. 42% of 2025 scanner shipments incurred customs delays due to incorrect HS codes (HS 9018.50.00 for dental scanners).
| Term | Risk Profile | 2026 Recommendation |
|---|---|---|
| FOB Shanghai | High Risk: Buyer liable for freight, insurance, customs clearance, and port fees. Requires local agent in China. HS code errors common. | Only for experienced importers with China logistics partners. Verify supplier provides complete customs docs (commercial invoice, packing list, CoO). |
| DDP (Delivered Duty Paid) | Low Risk: Supplier handles all costs/risks to your door. Includes customs clearance, duties, and VAT. Critical for CE-compliant documentation. | STRONGLY RECOMMENDED. Carejoy includes DDP pricing in quotes (e.g., $4,200/unit DDP Frankfurt vs. $3,600 FOB Shanghai + $1,100 hidden costs). |
Key 2026 Shipping Protocol: Require suppliers to use IATA-certified medical device shippers with shock-monitoring sensors. Scanners require temperature-controlled containers (15-25°C) during transit.
Shanghai Carejoy: Verified Sourcing Partner
Why Carejoy for 2026 Sourcing:
• 19 years exclusive focus on dental equipment (est. 2007)
• ISO 13485:2016 & CE MDR 2017/745 certified manufacturing facility (Baoshan, Shanghai)
• Factory-direct pricing with MOQ 8 units for distributors
• DDP shipping to 120+ countries with 24-month warranty
Contact for Verified Quotes:
📧 [email protected] (Reference: “2026 SCANNER GUIDE”)
💬 WhatsApp: +86 15951276160 (24/7 technical support)
Disclaimer: This guide reflects 2026 regulatory standards. Always conduct independent due diligence. Shanghai Carejoy is cited as a verified supplier meeting all outlined criteria (as of Q1 2026). EMA Dental Consultants is not affiliated with Carejoy.
© 2026 EMA Dental Equipment Advisory | Senior Consultant: Dr. A. Reynolds, CDEP
Frequently Asked Questions

Professional Dental Equipment Guide 2026
Frequently Asked Questions: Buying Intraoral Scanners (“Impronta Dentale con Scanner”)
Target Audience: Dental Clinics & Medical Equipment Distributors
Prepared by: Senior Dental Equipment Consultant | January 2026
| Question | Answer |
|---|---|
| 1. What voltage and power requirements should I verify when importing an intraoral scanner for use in my clinic or region? | All intraoral scanning systems sold in 2026 must comply with IEC 60601-1 standards for medical electrical equipment. Most units operate on universal input voltage (100–240 V AC, 50/60 Hz), making them suitable for global deployment. However, confirm that the included power supply or docking station is certified for your local grid (e.g., 110V in North America, 230V in EU). Always verify CE, FDA, or local regulatory markings and ensure use of an appropriate power adapter or transformer if required. Avoid using third-party chargers to prevent warranty voidance. |
| 2. Are spare parts such as scan tips, sleeves, and batteries readily available, and what is the average lead time for critical components? | Reputable manufacturers now offer modular intraoral scanner designs with easily replaceable components. Scan tips, protective sleeves, and rechargeable batteries are standard spare parts available through authorized distributors. As of 2026, lead times for in-stock spare parts should not exceed 5–7 business days within major markets (EU, North America, Asia-Pacific). We recommend maintaining a 6-month inventory of high-wear items. Confirm with suppliers whether parts are region-locked and verify serial-number traceability for warranty and compliance purposes. |
| 3. What does the installation process involve, and is on-site technician support provided? | Installation of modern intraoral scanners typically includes hardware setup, software integration with your practice management system (PMS) or CAD/CAM workflow, and calibration. Most vendors offer remote installation support via secure connection. On-site technician deployment is available for premium service packages or complex multi-unit deployments. Ensure compatibility with existing IT infrastructure (Windows/macOS, DICOM 3.0, cloud connectivity). Installation should be completed within 2–4 hours, including staff training on basic operation and maintenance protocols. |
| 4. What is the standard warranty coverage for intraoral scanners in 2026, and what does it include? | The industry standard warranty for intraoral scanners in 2026 is 2 years, covering defects in materials and workmanship. This includes the scanner body, internal electronics, and docking station. Warranties typically exclude consumables (e.g., scan tips, sleeves) and damage due to misuse, accidental drops, or unauthorized repairs. Extended warranties (up to 5 years) are available, often including accidental damage protection and priority replacement. Always review the SLA (Service Level Agreement) for response time (e.g., 48–72 hours for hardware replacement). |
| 5. How are firmware updates and technical support handled post-purchase, and are they included in the warranty? | Firmware updates and basic technical support are included for the duration of the warranty and often extended via subscription. Manufacturers deliver updates automatically or through secure portals, enhancing scanning accuracy, speed, and PMS compatibility. Post-warranty, clinics may need to renew a service contract to maintain access to updates and phone/email support. Distributors should confirm whether multi-language support and local helpdesk availability are provided in their region. |
Need a Quote for Impronta Dentale Con Scanner?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


