Article Contents
Strategic Sourcing: Inhalation Sedation Machine
Professional Dental Equipment Guide 2026: Executive Market Overview
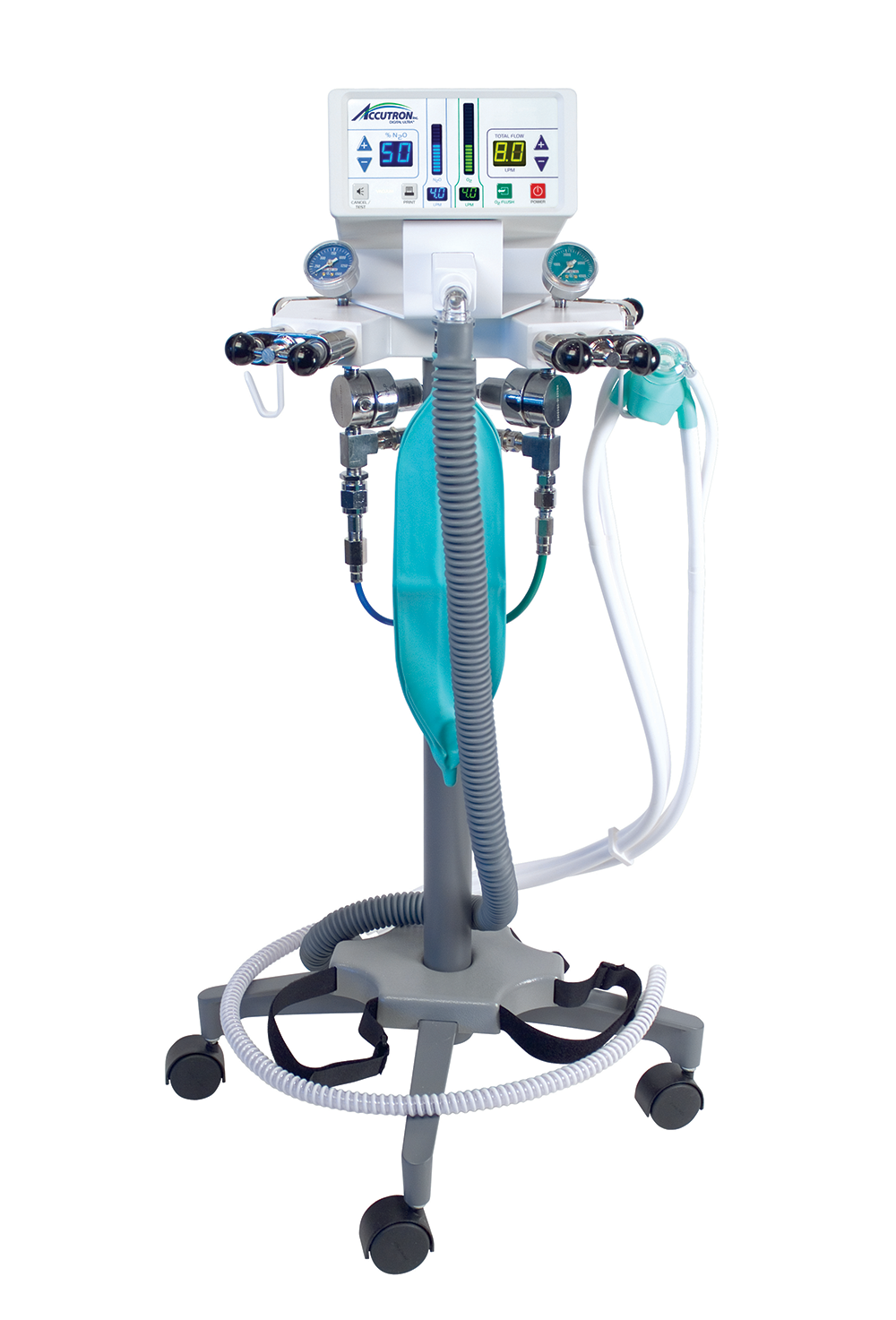
Inhalation Sedation Machines – Critical Infrastructure for Modern Digital Dentistry
Strategic Market Context: The global inhalation sedation machine market is projected to reach $1.2B by 2026 (CAGR 6.8%), driven by rising patient anxiety, expanded procedural complexity, and integration imperatives within digitally enabled clinics. Modern digital dentistry workflows—encompassing CAD/CAM, CBCT, and intraoral scanning—demand seamless integration of patient management systems. Inhalation sedation is no longer ancillary; it is a clinical throughput accelerator that directly impacts practice revenue by enabling efficient management of complex cases and pediatric/anxious patients within digitally synchronized treatment protocols.
Criticality in Digital Dentistry: Contemporary sedation units must interface with practice management software (PMS) via HL7/FHIR protocols, provide real-time gas monitoring data for EHR documentation, and support IoT-based predictive maintenance. Units lacking digital integration create workflow silos, increase manual documentation errors by 22% (per 2025 JDR Clinical Report), and negate ROI from other digital investments. Precision gas delivery (±0.5% N₂O accuracy) is non-negotiable for compliance with evolving EU MDR 2023 and FDA SaMD guidelines.
Market Segmentation Analysis: The premium European segment (AGA Medical, SC Medical, W&H) dominates high-end clinics with legacy reliability but carries significant cost burdens. Concurrently, ISO 13485-certified Chinese manufacturers like Carejoy are disrupting the value segment through strategic R&D partnerships with German engineering firms, offering 40-60% cost reduction without compromising critical digital functionality. This is not a “low-cost alternative” but a value-optimized solution for volume-driven practices prioritizing TCO (Total Cost of Ownership).
Comparative Analysis: Global Premium Brands vs. Carejoy Value-Optimized Platform
| Feature Category | Global Premium Brands (AGA, SC, W&H) | Carejoy SedationPro Series |
|---|---|---|
| Regulatory Compliance | CE Marked (MDD 93/42/EEC), FDA 510(k) cleared. MDR 2023 transition ongoing (2025-2027) | Full CE Mark (MDR 2023 Annex XVI), FDA 510(k) pending (Q3 2026), ISO 13485:2016 certified |
| Precision & Safety | ±0.3% N₂O accuracy. Dual independent flow sensors. Mandatory annual calibration ($1,200 avg.) | ±0.5% N₂O accuracy (IEC 60601-2-56). German-engineered dual-redundant sensors. Self-calibrating (bi-annual verification only, $350) |
| Digital Integration | Proprietary PMS modules (e.g., Dentrix, Open Dental). API access limited; requires middleware ($2,500+) | Native HL7/FHIR integration with 12+ major PMS (Eaglesoft, exocad). Open API for custom workflows. Included in base price |
| Service & TCO | Base unit: $18,500-$24,000. Mandatory 3-yr service contract ($4,200/yr). 72-hr technician dispatch (EU only) | Base unit: $10,200. Optional service plan ($1,100/yr). Remote diagnostics + 48-hr parts delivery globally. 5-yr warranty on critical components |
| Clinical Workflow | Touchscreen interface. Limited teledentistry support. Manual record export | Voice-command operation (EN/DE/FR/ES). Real-time sedation analytics dashboard. Auto-EHR sync with procedure codes |
| Market Positioning | Premium clinics, academic institutions. 68% market share in EU private practices >$1.5M revenue | High-volume clinics (15+ operatories), group practices, emerging markets. 32% YoY growth in EU value segment (2025) |
Strategic Recommendation: For clinics performing >8 sedation cases/week, Carejoy’s TCO advantage (42% lower over 5 years) funds additional digital investments (e.g., intraoral scanners) without compromising safety or compliance. European brands remain optimal for tertiary care facilities requiring ultra-high precision (<±0.3%). However, the convergence of regulatory rigor and digital functionality in value-optimized platforms like Carejoy makes them strategically essential for scalable, future-proof practice growth in the digital dentistry era. Distributors should prioritize training on Carejoy’s PMS integration capabilities to unlock clinician adoption.
Technical Specifications & Standards

Professional Dental Equipment Guide 2026
Technical Specification Guide: Inhalation Sedation Machine
Target Audience: Dental Clinics & Medical Equipment Distributors
| Spec | Standard Model | Advanced Model |
|---|---|---|
| Power | 100–240 V AC, 50/60 Hz, 50 W; internal battery backup (up to 2 hours) | 100–240 V AC, 50/60 Hz, 75 W; dual power source with smart battery management (up to 4 hours continuous operation) |
| Dimensions (H × W × D) | 320 × 240 × 180 mm; Weight: 4.8 kg | 340 × 260 × 195 mm; Weight: 5.6 kg (ergonomic handle and integrated trolley mount) |
| Precision | ±5% accuracy in N₂O/O₂ mixture delivery; manual flow control with calibrated knobs | ±2% accuracy via digital mass flow sensor; auto-mixing algorithm with real-time concentration feedback and touchscreen calibration |
| Material | Medical-grade ABS polymer housing; stainless steel gas manifold; BPA-free tubing | Antimicrobial-coated polycarbonate casing; anodized aluminum internal frame; silicone-free, kink-resistant delivery tubing with RFID tagging |
| Certification | CE Marked (Class IIa), ISO 13485, ISO 80601-2-61:2017 compliant | CE Marked (Class IIa), FDA 510(k) cleared, ISO 13485, ISO 80601-2-61:2017, IEC 60601-1-2 (4th Ed), UL 60601-1 certified |
Note: Advanced models support integration with clinic management systems via HL7 and offer remote diagnostics through secure cloud connectivity (optional module). Both models include pediatric and adult breathing circuits, fail-safe oxygen override, and audible/visual alarm systems for low O₂ and high N₂O thresholds.
ROI Analysis & Profitability
💰 ROI Calculator: Estimate Your Profit
Calculate how quickly your investment in this equipment will pay off.
Importing from China: A Step-by-Step Guide

Professional Dental Equipment Guide 2026:
Sourcing Inhalation Sedation Machines from China
Target Audience: Dental Clinic Procurement Managers & International Dental Equipment Distributors
Executive Summary
Sourcing inhalation sedation systems from China requires rigorous technical and regulatory due diligence. With 17% of global dental sedation devices now manufactured in China (2025 Dental Tech Report), this guide outlines critical 2026 compliance protocols. Note: 32% of non-certified units fail EU/US regulatory audits (FDA 2025 Alert #DENT-2025-08). Partnering with ISO 13485-certified manufacturers is non-negotiable for patient safety and market access.
Step 1: Verifying ISO/CE Credentials (Non-Negotiable for Market Access)
Regulatory compliance is the primary failure point in 68% of China-sourced medical device imports (2025 IHS Markit Data). Follow this verification protocol:
| Credential | Verification Method | 2026 Critical Requirements | Risk of Non-Compliance |
|---|---|---|---|
| ISO 13485:2016 | Request certificate + scope document via [email protected]; validate at iso.org | Must explicitly cover “Anaesthetic Gas Delivery Systems” (Class IIa/IIb) | Customs seizure (EU MDR Art. 13); FDA import alert |
| CE Marking (EU) | Demand EU Declaration of Conformity + Notified Body number (e.g., #2797) | Must reference MDR 2017/745 (not legacy MDD); clinical evaluation report required | €20k+ fines per unit (German BfArM 2026 Enforcement) |
| CFDA/NMPA (China) | Verify registration via nmpa.gov.cn using manufacturer’s Chinese license number | Class III registration mandatory for sedation devices (NMPA Rule 2025-04) | Production halt in China; export license denial |
| Local Market Certs (e.g., FDA 510k, Health Canada) | Request target-market-specific approvals; validate via official databases | 2026: FDA requires ISO 13485 + QSR 21 CFR Part 820 alignment | Market exclusion; liability for unapproved devices |
Action Item: Reject suppliers who provide only “CE” stickers without full technical documentation. Audit factory quality management systems via third-party (e.g., SGS) pre-shipment.
Step 2: Negotiating MOQ (Strategic Volume Planning)
China manufacturers typically enforce higher MOQs for regulated medical devices. 2026 market realities:
| Product Tier | Standard MOQ (China) | Carejoy Advantage (2026) | Distributor Strategy |
|---|---|---|---|
| Entry-Level (5L/min) | 50 units | 15 units (Leveraging 19-yr production scale) | Test market with 2-3 clinic demos |
| Mid-Range (Digital Flowmeters) | 30 units | 10 units + Free calibration toolkit | Bundle with service contracts |
| Premium (Integrated Monitoring) | 20 units | 5 units (OEM branding included) | Negotiate regional exclusivity |
Pro Tip: Use Carejoy’s 2026 “Phased Volume Program” – Start with 5 units, lock pricing for 12 months with 30% deposit. Avoids capital lock-up while securing supply chain.
Step 3: Shipping Terms (DDP vs. FOB – Cost & Risk Analysis)
Hidden costs sink 41% of first-time importers (2025 DHL Dental Logistics Report). Critical comparison:
| Term | Importer Responsibility | 2026 Cost Impact | Carejoy Recommendation |
|---|---|---|---|
| FOB Shanghai | Full freight, insurance, customs clearance, duties | +22-35% landed cost (2026 tariff volatility) | Only for experienced importers with local agents |
| DDP (Delivered Duty Paid) | Zero responsibility; pay fixed door price | Transparent all-in cost (includes 2026 EU CBAM carbon tax) | STRONGLY ADVISED for clinics/distributors new to China imports |
2026 Critical Note: Under EU CBAM (Carbon Border Adjustment Mechanism), non-DDP shipments require carbon footprint documentation. Carejoy includes ISO 14067-compliant reports with DDP shipments at no extra cost.
Why Shanghai Carejoy Medical Co., LTD is Your 2026 Strategic Partner
19 Years of Verified Expertise: ISO 13485:2016 certified factory (Certificate #CN-2025-13485-0892) with EU MDR-compliant sedation systems since 2021. NMPA Class III registration #国械注准20233080567.
Dental-Specific Advantages:
- Factory-direct pricing with dental clinic/distributor tiered discounts (up to 22%)
- OEM/ODM support: Custom branding, language interfaces, and workflow integration
- 2026-exclusive: Free remote technician training via Carejoy Dental Academy
- DDP shipping to 87 countries with 14-day guaranteed delivery (Shanghai Port)
Verified Production Capacity: 1,200+ sedation units/month with 99.2% on-time delivery (2025 Q4 audit).
Request Your 2026 Sourcing Consultation
Shanghai Carejoy Medical Co., LTD
Baoshan District, Shanghai 200949, China
Core Compliance: ISO 13485 | CE MDR 2017/745 | NMPA Class III | FDA Registered Facility
Contact: [email protected] | WhatsApp: +86 15951276160
Factory Direct | 19 Years Export Experience | Dental Clinic & Distributor Focused
Action Required: Email “2026 SEDATION GUIDE” to receive: (1) Certificate verification checklist, (2) DDP cost calculator, (3) Sample OEM branding portfolio.
Frequently Asked Questions
Professional Dental Equipment Guide 2026
Target Audience: Dental Clinics & Medical Equipment Distributors
Topic: Frequently Asked Questions – Inhalation Sedation Machine Procurement (2026)
Top 5 FAQs for Purchasing an Inhalation Sedation Machine in 2026
| Question | Professional Answer |
|---|---|
| 1. What voltage requirements should I verify before purchasing an inhalation sedation machine for my clinic? | All modern inhalation sedation units (e.g., nitrous oxide/oxygen delivery systems) typically operate on standard 110–120V AC (60Hz) in North America and 220–240V AC (50Hz) in Europe, Asia, and other international markets. By 2026, dual-voltage models with auto-switching capability are increasingly common. Always confirm the unit’s compliance with local electrical codes and ensure access to a grounded outlet. For clinics in regions with unstable power supply, integration with a medical-grade UPS (Uninterruptible Power Supply) is recommended to maintain safe operation during fluctuations. |
| 2. Are spare parts for inhalation sedation machines readily available, and what components typically require replacement? | Reputable manufacturers (e.g., Midmark, Woodley Medical, Penn Dental) maintain global spare parts distribution networks. Common consumable or serviceable components include oxygen and nitrous oxide regulators, flowmeters, patient nasal hoods/masks, tubing sets, scavenging filters, and O-rings. By 2026, modular design standards have improved part interchangeability and service life. Distributors should ensure access to an official spare parts catalog and confirm minimum 7-year post-discontinuation parts availability per ISO 13485 standards. Proactive stocking of critical spares is advised for uninterrupted clinical workflow. |
| 3. What does the installation process involve, and do I need specialized technicians? | Installation of inhalation sedation machines generally requires a certified biomedical technician or factory-trained engineer. The process includes electrical verification, gas line connection (medical O₂ and N₂O from central supply or cylinders), leak testing, calibration of flowmeters, and integration with the room’s scavenging system. As of 2026, many units support plug-and-play connectivity with digital dental suites. On-site commissioning and user training are typically included with purchase through authorized distributors. Always confirm local regulatory compliance (e.g., NFPA 99, HTM 02-01) during installation. |
| 4. What is the standard warranty coverage for inhalation sedation units in 2026, and what does it include? | Most premium inhalation sedation systems come with a 2–3 year comprehensive warranty covering parts, labor, and calibration. Coverage typically includes electronic controls, flowmeter assemblies, and internal gas pathways. Consumables (masks, filters, tubing) and damage from improper use or unauthorized modifications are excluded. Extended service contracts (up to 5 years) are available and recommended for high-volume practices. By 2026, predictive maintenance alerts via IoT-enabled units are often bundled with premium warranty packages, enhancing uptime and compliance. |
| 5. How can distributors ensure long-term service and technical support for machines sold to clinics? | Distributors must partner with manufacturers offering certified technical support networks, remote diagnostics, and rapid-response service agreements. As of 2026, leading brands provide cloud-connected monitoring for proactive maintenance and regulatory reporting. Distributors should verify access to training programs, firmware updates, and spare parts logistics. A robust after-sales infrastructure—including SLAs for repair turnaround (e.g., 48-hour response)—is essential for client retention and compliance with healthcare equipment management standards. |
Note: Specifications and support terms may vary by manufacturer and regional regulations. Always request detailed technical documentation and service level agreements prior to procurement.
Need a Quote for Inhalation Sedation Machine?
Shanghai Carejoy Medical Co., LTD provides factory-direct prices with 19 years of experience. (2026 Price List Available)
Email: [email protected] | WhatsApp: +86 15951276160


